| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:30:14 UTC |
|---|
| Update Date | 2016-11-09 01:18:24 UTC |
|---|
| Accession Number | CHEM027107 |
|---|
| Identification |
|---|
| Common Name | 7-Hydroxy-3,3',4',5,6,8-hexamethoxyflavone |
|---|
| Class | Small Molecule |
|---|
| Description | 7-Hydroxy-3,3',4',5,6,8-hexamethoxyflavone is found in citrus. 7-Hydroxy-3,3',4',5,6,8-hexamethoxyflavone is a constituent of the peel of tangerine (Citrus sp.). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
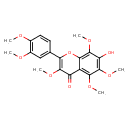
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3,4-Dimethoxyphenyl)-7-hydroxy-3,5,6,8-tetramethoxy-4H-1-benzopyran-4-one | HMDB | | 7-Hydroxy-3,5,6,8,3',4'-hexamethoxyflavone | HMDB |
|
|---|
| Chemical Formula | C21H22O9 |
|---|
| Average Molecular Mass | 418.394 g/mol |
|---|
| Monoisotopic Mass | 418.126 g/mol |
|---|
| CAS Registry Number | 185678-89-1 |
|---|
| IUPAC Name | 2-(3,4-dimethoxyphenyl)-7-hydroxy-3,5,6,8-tetramethoxy-4H-chromen-4-one |
|---|
| Traditional Name | 2-(3,4-dimethoxyphenyl)-7-hydroxy-3,5,6,8-tetramethoxychromen-4-one |
|---|
| SMILES | COC1=C(OC)C=C(C=C1)C1=C(OC)C(=O)C2=C(OC)C(OC)=C(O)C(OC)=C2O1 |
|---|
| InChI Identifier | InChI=1S/C21H22O9/c1-24-11-8-7-10(9-12(11)25-2)16-19(27-4)14(22)13-17(26-3)20(28-5)15(23)21(29-6)18(13)30-16/h7-9,23H,1-6H3 |
|---|
| InChI Key | LIHVLVGTXLTMAQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 8-o-methylated flavonoids. These are flavonoids with methoxy groups attached to the C8 atom of the flavonoid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | O-methylated flavonoids |
|---|
| Direct Parent | 8-O-methylated flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3p-methoxyflavonoid-skeleton
- 3-methoxyflavonoid-skeleton
- 4p-methoxyflavonoid-skeleton
- 5-methoxyflavonoid-skeleton
- 6-methoxyflavonoid-skeleton
- 8-methoxyflavonoid-skeleton
- 7-hydroxyflavonoid
- Flavone
- Hydroxyflavonoid
- 3-methoxychromone
- Chromone
- O-dimethoxybenzene
- Dimethoxybenzene
- Benzopyran
- 1-benzopyran
- Phenoxy compound
- Methoxybenzene
- Anisole
- Phenol ether
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Monocyclic benzene moiety
- Pyran
- Vinylogous ester
- Heteroaromatic compound
- Ether
- Organoheterocyclic compound
- Oxacycle
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f79-0039400000-735720c1f30378d90d38 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0200-1010900000-ae14607ef00a56b524cc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-2f4af581863816724cef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0001900000-a34eb4926b0ee69ddbfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0129100000-731e6c6199a1f753fd8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-8eae570c5755a411434b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0006900000-826a4fef3128a6896da5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dm-0429000000-c4c4adf52c18cf309ac2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-a12148ee24803ea18836 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000900000-ad62db498bcaebea9a28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00or-1191300000-5f096beead1d96d9393d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-0a6930dd8545a0febcdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0031900000-cc8cafc7600cfaa2c713 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0550-2893100000-cb23a1b3346d1830dd4d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032785 |
|---|
| FooDB ID | FDB010756 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00013378 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8020612 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9844898 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|