| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:28:46 UTC |
|---|
| Update Date | 2016-11-09 01:18:23 UTC |
|---|
| Accession Number | CHEM027071 |
|---|
| Identification |
|---|
| Common Name | Phloroacetophenone 6'-[xylosyl-(1->6)-glucoside] |
|---|
| Class | Small Molecule |
|---|
| Description | Phloroacetophenone 6'-[xylosyl-(1->6)-glucoside] is found in fats and oils. Phloroacetophenone 6'-[xylosyl-(1->6)-glucoside] is a constituent of Sapium sebiferum (Chinese tallowtree). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
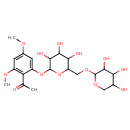
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C21H30O13 |
|---|
| Average Molecular Mass | 490.455 g/mol |
|---|
| Monoisotopic Mass | 490.169 g/mol |
|---|
| CAS Registry Number | 87432-28-8 |
|---|
| IUPAC Name | 1-{2,4-dimethoxy-6-[(3,4,5-trihydroxy-6-{[(3,4,5-trihydroxyoxan-2-yl)oxy]methyl}oxan-2-yl)oxy]phenyl}ethan-1-one |
|---|
| Traditional Name | 1-{2,4-dimethoxy-6-[(3,4,5-trihydroxy-6-{[(3,4,5-trihydroxyoxan-2-yl)oxy]methyl}oxan-2-yl)oxy]phenyl}ethanone |
|---|
| SMILES | COC1=CC(OC)=C(C(C)=O)C(OC2OC(COC3OCC(O)C(O)C3O)C(O)C(O)C2O)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H30O13/c1-8(22)14-11(30-3)4-9(29-2)5-12(14)33-21-19(28)17(26)16(25)13(34-21)7-32-20-18(27)15(24)10(23)6-31-20/h4-5,10,13,15-21,23-28H,6-7H2,1-3H3 |
|---|
| InChI Key | BAYBVSSLAYYEKP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- Alkyl-phenylketone
- Disaccharide
- O-glycosyl compound
- Dimethoxybenzene
- M-dimethoxybenzene
- Acetophenone
- Phenylketone
- Phenoxy compound
- Anisole
- Benzoyl
- Phenol ether
- Aryl ketone
- Aryl alkyl ketone
- Methoxybenzene
- Alkyl aryl ether
- Oxane
- Benzenoid
- Monocyclic benzene moiety
- Ketone
- Secondary alcohol
- Polyol
- Ether
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-024i-2332900000-673a5b2836838c28f613 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014i-3234119000-dae758d72233400643fa | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006t-0901800000-5188291cfe5a2d01e5e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0901000000-47ea2af214410e2e9f95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-1900000000-9db4f3f61066300e671b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-1921800000-8a70a423f560afa6b2ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900200000-ea87d0eb880e16096954 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-3900000000-eb2df55b6cb8157e0d31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0102900000-88b4153faf40916487d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056v-2309100000-fa3ada539e661240e914 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fr6-3901000000-14132834506b492193bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0902200000-153397c3b5655cb11156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003s-0900000000-737056300ea1acc820e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-1910000000-8b65acf4a80fa620a9f3 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032743 |
|---|
| FooDB ID | FDB010705 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 169408 |
|---|
| PubChem Compound ID | 131751293 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|