| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:28:31 UTC |
|---|
| Update Date | 2016-11-09 01:18:23 UTC |
|---|
| Accession Number | CHEM027064 |
|---|
| Identification |
|---|
| Common Name | 3,4-Dimethoxy-3,4-desmethylenecubebin |
|---|
| Class | Small Molecule |
|---|
| Description | (8R,8'R,9S)-9-Hydroxy-3,4-dimethoxy-3',4'-methylenoxy-9,9'-epoxylignan is a constituent of Piper chaba (Javanese long pepper) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
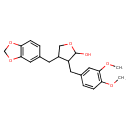
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-Dimethoxy-3,4-desmethylenecubebin | HMDB |
|
|---|
| Chemical Formula | C21H24O6 |
|---|
| Average Molecular Mass | 372.412 g/mol |
|---|
| Monoisotopic Mass | 372.157 g/mol |
|---|
| CAS Registry Number | 77533-71-2 |
|---|
| IUPAC Name | 4-(2H-1,3-benzodioxol-5-ylmethyl)-3-[(3,4-dimethoxyphenyl)methyl]oxolan-2-ol |
|---|
| Traditional Name | 4-(2H-1,3-benzodioxol-5-ylmethyl)-3-[(3,4-dimethoxyphenyl)methyl]oxolan-2-ol |
|---|
| SMILES | COC1=C(OC)C=C(CC2C(CC3=CC4=C(OCO4)C=C3)COC2O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C21H24O6/c1-23-17-5-3-14(9-19(17)24-2)8-16-15(11-25-21(16)22)7-13-4-6-18-20(10-13)27-12-26-18/h3-6,9-10,15-16,21-22H,7-8,11-12H2,1-2H3 |
|---|
| InChI Key | FDXPFHFRXMBVEU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzylbutyrolactols. These are lignan compounds containing a 3,4-dibenzyloxolan-2-ol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Furanoid lignans |
|---|
| Sub Class | Tetrahydrofuran lignans |
|---|
| Direct Parent | Dibenzylbutyrolactols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzylbutyrolactol
- Dimethoxybenzene
- O-dimethoxybenzene
- Benzodioxole
- Methoxybenzene
- Anisole
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Tetrahydrofuran
- Hemiacetal
- Ether
- Organoheterocyclic compound
- Acetal
- Oxacycle
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01pc-0915000000-dbd2878807bf102b561d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01ri-4902200000-6e1ac9705ea62af96194 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0219000000-2fd1d66c280383e92dbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0749000000-84ed6b70b114bd08dec1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wmj-1910000000-31390de8c1464e607618 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-c346791bc119cfa3e246 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fr-0109000000-cfe3c7059181263b849d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0n4j-0296000000-f35e807f02e65565301e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0109000000-6cb45a344a528d9b05b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fe0-0926000000-df9888744b6dff2a2abd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00du-2916000000-cf1dbc6b1c46db061b28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-2eb2de302a2fafba3411 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-0019000000-b88b4efcc55b1c75c021 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-1595000000-7f69881c44ab783af5c5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032733 |
|---|
| FooDB ID | FDB013341 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013477 |
|---|
| ChEBI ID | 175785 |
|---|
| PubChem Compound ID | 14189058 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|