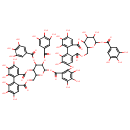| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:28:04 UTC |
|---|
| Update Date | 2016-11-09 01:18:23 UTC |
|---|
| Accession Number | CHEM027053 |
|---|
| Identification |
|---|
| Common Name | Heterophylliin F |
|---|
| Class | Small Molecule |
|---|
| Description | Heterophylliin F is found in nuts. Heterophylliin F is isolated from leaves of Corylus heterophylla (Siberian filbert). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4,5,21,22,23-Hexahydroxy-8,18-dioxo-11,12-bis(3,4,5-trihydroxybenzoyloxy)-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2,4,6,19,21-hexaen-13-yl 2-{[3,4,5,11,12,22,23-heptahydroxy-8,18-dioxo-13-(3,4,5-trihydroxybenzoyloxy)-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2,4,6,19,21-hexaen-21-yl]oxy}-3,4,5-trihydroxybenzoic acid | HMDB |
|
|---|
| Chemical Formula | C68H50O44 |
|---|
| Average Molecular Mass | 1571.098 g/mol |
|---|
| Monoisotopic Mass | 1570.167 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3,4,5,21,22,23-hexahydroxy-8,18-dioxo-11,12-bis(3,4,5-trihydroxybenzoyloxy)-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2,4,6,19,21-hexaen-13-yl 2-{[3,4,5,11,12,22,23-heptahydroxy-8,18-dioxo-13-(3,4,5-trihydroxybenzoyloxy)-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2,4,6,19,21-hexaen-21-yl]oxy}-3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | 3,4,5,21,22,23-hexahydroxy-8,18-dioxo-11,12-bis(3,4,5-trihydroxybenzoyloxy)-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2,4,6,19,21-hexaen-13-yl 2-{[3,4,5,11,12,22,23-heptahydroxy-8,18-dioxo-13-(3,4,5-trihydroxybenzoyloxy)-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2,4,6,19,21-hexaen-21-yl]oxy}-3,4,5-trihydroxybenzoate |
|---|
| SMILES | OC1C(O)C2OC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(OC4=C(C=C(O)C(O)=C4O)C(=O)OC4OC5COC(=O)C6=CC(O)=C(O)C(O)=C6C6=C(O)C(O)=C(O)C=C6C(=O)OC5C(OC(=O)C5=CC(O)=C(O)C(O)=C5)C4OC(=O)C4=CC(O)=C(O)C(O)=C4)C=C3C(=O)OCC2OC1OC(=O)C1=CC(O)=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C68H50O44/c69-22-1-14(2-23(70)39(22)79)59(94)109-57-56-34(13-103-62(97)17-7-28(75)42(82)47(87)35(17)36-19(65(100)108-56)9-30(77)43(83)48(36)88)106-68(58(57)110-60(95)15-3-24(71)40(80)25(72)4-15)112-66(101)21-10-31(78)45(85)51(91)54(21)104-32-11-20-38(50(90)46(32)86)37-18(8-29(76)44(84)49(37)89)64(99)107-55-33(12-102-63(20)98)105-67(53(93)52(55)92)111-61(96)16-5-26(73)41(81)27(74)6-16/h1-11,33-34,52-53,55-58,67-93H,12-13H2 |
|---|
| InChI Key | MCGBUJRCIHNSPF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydrolyzable tannins. These are tannins with a structure characterized by either of the following models. In model 1, the structure contains galloyl units (in some cases, shikimic acid units) that are linked to diverse polyol carbohydrate-, catechin-, or triterpenoid units. In model 2, contains at least two galloyl units C-C coupled to each other, and do not contain a glycosidically linked catechin unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Tannins |
|---|
| Sub Class | Hydrolyzable tannins |
|---|
| Direct Parent | Hydrolyzable tannins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrolyzable tannin
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Dihydroxybenzoic acid
- Diaryl ether
- Benzoate ester
- Benzenetriol
- Benzoic acid or derivatives
- Pyrogallol derivative
- Phenoxy compound
- Phenol ether
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Oxane
- Monocyclic benzene moiety
- Monosaccharide
- Benzenoid
- Lactone
- Carboxylic acid ester
- Secondary alcohol
- 1,2-diol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Carboxylic acid derivative
- Ether
- Organic oxygen compound
- Alcohol
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0700454930-8dfb427485a6f62f4ffb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0901132510-34bfa33b09119c559cf7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v4i-0900134100-d97914cfe8b584f63b1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0606291730-c6ed2e1b18cb3a195e76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2901142220-3a71c0950813a2962f56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0901001000-f83cb9e4afd2168e0ae4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gb9-0101196540-4ce3f442da1a69817ec3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gb9-1425290200-079dd943334b52881f66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02di-1900260000-9e26c16c0d48462c8cb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0003980300-d4d1885c39d8e7509e70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0312591300-9e1de665457de5dfdcea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-2904473000-6b61e8954ddcff1c7b05 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032720 |
|---|
| FooDB ID | FDB010681 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751290 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|