| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:27:28 UTC |
|---|
| Update Date | 2016-11-09 01:18:23 UTC |
|---|
| Accession Number | CHEM027040 |
|---|
| Identification |
|---|
| Common Name | Ammonium alum |
|---|
| Class | Small Molecule |
|---|
| Description | It is used in foods as a buffer and neutralizing agent
Ammonium alum (NH4Al(SO4)2·12H2O) or ammonium aluminium sulfate dodecahydrate is a white crystalline double sulfate of aluminium, used in water purification, in vegetable glues, in porcelain cements, in natural deodorants and in tanning, dyeing and in fireproofing textiles.; The pH of the solution resulting from the topical application of ammonium alum with perspiration is typically in the slightly acid range, from 4 to 5. It is a popular deodorant because of its high availability and low cost. A 120 gram stone lasts for at least a year of daily usage - much longer than other deodorants and antiperspirants. It is also hypoallergenic and non-staining. Potassium alum is also used for this purpose. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
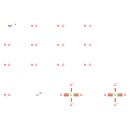
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Aluminum ammonium sulfate (3:1:3) | MeSH | | Aluminum ammonium sulfate | MeSH | | Aluminum ammonium sulfate (2:1:1) dodecahydrate | MeSH | | Aluminum sulfate ammonium salt | MeSH |
|
|---|
| Chemical Formula | AlH28NO20S2 |
|---|
| Average Molecular Mass | 453.329 g/mol |
|---|
| Monoisotopic Mass | 453.046 g/mol |
|---|
| CAS Registry Number | 7784-26-1 |
|---|
| IUPAC Name | aluminium(3+) ion ammonium dodecahydrate disulfate |
|---|
| Traditional Name | aluminium(3+) ion ammonium dodecahydrate disulfate |
|---|
| SMILES | [NH4+].O.O.O.O.O.O.O.O.O.O.O.O.[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/Al.H3N.2H2O4S.12H2O/c;;2*1-5(2,3)4;;;;;;;;;;;;/h;1H3;2*(H2,1,2,3,4);12*1H2/q+3;;;;;;;;;;;;;;;/p-3 |
|---|
| InChI Key | WZUKKIPWIPZMAS-UHFFFAOYSA-K |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as post-transition metal sulfates. These are inorganic compounds in which the largest oxoanion is sulfate, and in which the heaviest atom not in an oxoanion is a post-transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Post-transition metal oxoanionic compounds |
|---|
| Sub Class | Post-transition metal sulfates |
|---|
| Direct Parent | Post-transition metal sulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Post-transition metal sulfate
- Inorganic post-transition metal salt
- Inorganic oxide
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000900000-f72cbb72b481171cf831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000900000-f72cbb72b481171cf831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0000900000-f72cbb72b481171cf831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-4bcf09608e8742594697 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000900000-4bcf09608e8742594697 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0000900000-4bcf09608e8742594697 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032701 |
|---|
| FooDB ID | FDB010659 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ammonium aluminium sulfate |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 62668 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|