| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:27:20 UTC |
|---|
| Update Date | 2016-11-09 01:18:23 UTC |
|---|
| Accession Number | CHEM027037 |
|---|
| Identification |
|---|
| Common Name | 3-(2-Methylpropanoyloxy)-8-(2-methylbutanoyloxy)-9,10-epoxy-p-mentha-1,3,5-triene |
|---|
| Class | Small Molecule |
|---|
| Description | 3-(2-Methylpropanoyloxy)-8-(2-methylbutanoyloxy)-9,10-epoxy-p-mentha-1,3,5-triene is found in fats and oils. 3-(2-Methylpropanoyloxy)-8-(2-methylbutanoyloxy)-9,10-epoxy-p-mentha-1,3,5-triene is a constituent of various plant species including Madia sativa (Chile tarweed) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
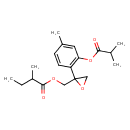
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2-{4-methyl-2-[(2-methylpropanoyl)oxy]phenyl}oxiran-2-yl)methyl 2-methylbutanoic acid | HMDB |
|
|---|
| Chemical Formula | C19H26O5 |
|---|
| Average Molecular Mass | 334.407 g/mol |
|---|
| Monoisotopic Mass | 334.178 g/mol |
|---|
| CAS Registry Number | 22518-07-6 |
|---|
| IUPAC Name | (2-{4-methyl-2-[(2-methylpropanoyl)oxy]phenyl}oxiran-2-yl)methyl 2-methylbutanoate |
|---|
| Traditional Name | (2-{4-methyl-2-[(2-methylpropanoyl)oxy]phenyl}oxiran-2-yl)methyl 2-methylbutanoate |
|---|
| SMILES | CCC(C)C(=O)OCC1(CO1)C1=C(OC(=O)C(C)C)C=C(C)C=C1 |
|---|
| InChI Identifier | InChI=1S/C19H26O5/c1-6-14(5)18(21)22-10-19(11-23-19)15-8-7-13(4)9-16(15)24-17(20)12(2)3/h7-9,12,14H,6,10-11H2,1-5H3 |
|---|
| InChI Key | DBEFONQGRSUFQO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenol esters. These are aromatic compounds containing a benzene ring substituted by a hydroxyl group and an ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol esters |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenol esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenol ester
- Phenoxy compound
- Fatty acid ester
- Toluene
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052g-9321000000-b4b70148eb3762f68973 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9187000000-ad129e62a02bb3f3cd59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ad-9141000000-e70a2e7a70454c0a58b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-a75204728b469aa69064 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-053r-3927000000-f50324b9b3dc745ada59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kai-9523000000-4cbbc9b581fd3320f490 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aou-8900000000-47ca776a604bf58b7cf4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0159000000-f8ea17d8bafc920dce91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-9682000000-a90ff5cd5a6f78e042df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-7911000000-bce5b1fb07e2adac8ae5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001j-3896000000-8c80faee2ef75f820bcb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001r-9421000000-893a2cf0cea623f6bec6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05aj-9610000000-b084324ec8a69049c0ae | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032699 |
|---|
| FooDB ID | FDB010656 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 314387 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 354159 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|