| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:26:19 UTC |
|---|
| Update Date | 2016-11-09 01:18:22 UTC |
|---|
| Accession Number | CHEM027010 |
|---|
| Identification |
|---|
| Common Name | 2-[(5-Methylsulfinyl)-4-penten-2-ynylidene]-1,6-dioxaspiro[4.4]non-3-ene |
|---|
| Class | Small Molecule |
|---|
| Description | (7Z,11E)-2-(5-Methylsulfinyl-2-pentyn-4-enylidene)-1,6-dioxaspiro[4.4]non-3-ene is found in herbs and spices. (7Z,11E)-2-(5-Methylsulfinyl-2-pentyn-4-enylidene)-1,6-dioxaspiro[4.4]non-3-ene is isolated from Chrysanthemum coronarium (chop-suey greens |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
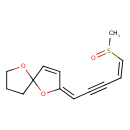
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-[(5-Methylsulphinyl)-4-penten-2-ynylidene]-1,6-dioxaspiro[4.4]non-3-ene | Generator | | (2E)-2-[(4Z)-5-Methanesulphinylpent-4-en-2-yn-1-ylidene]-1,6-dioxaspiro[4.4]non-3-ene | HMDB | | (7Z,11E)-2-(5-Methylsulphinyl-2-pentyn-4-enylidene)-1,6-dioxaspiro[4.4]non-3-ene | HMDB |
|
|---|
| Chemical Formula | C13H14O3S |
|---|
| Average Molecular Mass | 250.313 g/mol |
|---|
| Monoisotopic Mass | 250.066 g/mol |
|---|
| CAS Registry Number | 124460-81-7 |
|---|
| IUPAC Name | (2E)-2-[(4Z)-5-methanesulfinylpent-4-en-2-yn-1-ylidene]-1,6-dioxaspiro[4.4]non-3-ene |
|---|
| Traditional Name | (2E)-2-[(4Z)-5-methanesulfinylpent-4-en-2-yn-1-ylidene]-1,6-dioxaspiro[4.4]non-3-ene |
|---|
| SMILES | CS(=O)\C=C/C#C\C=C1\OC2(CCCO2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C13H14O3S/c1-17(14)11-4-2-3-6-12-7-9-13(16-12)8-5-10-15-13/h4,6-7,9,11H,5,8,10H2,1H3/b11-4-,12-6+ |
|---|
| InChI Key | HGPOBYCDHSNTNP-QFQHGIJPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ketals. These are acetals derived from ketones by replacement of the oxo group by two hydrocarbyloxy groups R2C(OR)2 ( R not Hydrogen ). This term, once abandoned, has been reinstated as a subclass of acetals. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Ethers |
|---|
| Direct Parent | Ketals |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketal
- Tetrahydrofuran
- Dihydrofuran
- Sulfoxide
- Oxacycle
- Organoheterocyclic compound
- Sulfinyl compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9450000000-4d6bbf1ecf79447b4da9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-3590000000-14cbbe48015e7afd0541 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-4930000000-a47c1e80e566bb3d9663 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9600000000-a6b2d35d16a216ca905c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-9240000000-ca83b01d732a28b9fd6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-e0b9d2533e1b718ac8a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9000000000-0cf9f5d193fc8193cf5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-2090000000-66fc63844ccbaa6d768c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9380000000-92214656543836aeaa2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fu-7910000000-c136aa016eb70c2f00b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0490000000-2356178aa842ae153e07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-07vu-1960000000-3e636497ef8f568ddaaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdr-3910000000-1e8147469545899e69f8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032670 |
|---|
| FooDB ID | FDB013426 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10275814 |
|---|
| ChEBI ID | 174290 |
|---|
| PubChem Compound ID | 14540702 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|