| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:26:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:22 UTC |
|---|
| Accession Number | CHEM027004 |
|---|
| Identification |
|---|
| Common Name | Corchorifatty acid A |
|---|
| Class | Small Molecule |
|---|
| Description | Corchorifatty acid C is found in green vegetables. Corchorifatty acid C is a constituent of Corchorus olitorius (Jew's mallow) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
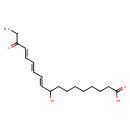
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (10E,12E,14E)-9-Hydroxy-16-oxooctadeca-10,12,14-trienoate | HMDB |
|
|---|
| Chemical Formula | C18H28O4 |
|---|
| Average Molecular Mass | 308.413 g/mol |
|---|
| Monoisotopic Mass | 308.199 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (10E,12E,14E)-9-hydroxy-16-oxooctadeca-10,12,14-trienoic acid |
|---|
| Traditional Name | (10E,12E,14E)-9-hydroxy-16-oxooctadeca-10,12,14-trienoic acid |
|---|
| SMILES | CCC(=O)\C=C\C=C\C=C\C(O)CCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H28O4/c1-2-16(19)12-8-6-7-10-14-17(20)13-9-4-3-5-11-15-18(21)22/h6-8,10,12,14,17,20H,2-5,9,11,13,15H2,1H3,(H,21,22)/b7-6+,12-8+,14-10+ |
|---|
| InChI Key | FCCFXOHVERVHQR-KDXRDGMUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Lineolic acids and derivatives |
|---|
| Direct Parent | Lineolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Octadecanoid
- Long-chain fatty acid
- Hydroxy fatty acid
- Keto fatty acid
- Fatty acid
- Unsaturated fatty acid
- Acryloyl-group
- Enone
- Alpha,beta-unsaturated ketone
- Secondary alcohol
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-3950000000-54407b57dc7154d1e55a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-009i-9445400000-ac237e9095a7fccc0163 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0596-0092000000-5b0e3534d2b6f844d499 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fu-2390000000-9ddaf57e2207824d4f72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uym-9240000000-ef791194db28c7155ebf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1059000000-f5c754681f3bb127063f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2193000000-90d993f030bf43e2c255 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9330000000-0e65ceb328b2a121bb23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0029000000-716620ad958d0ec01724 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-3193000000-5292f8769e10b5c2d792 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9860000000-018a172c0ac98fa64e40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0291000000-d4bf848ead7bb0243b8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2970000000-90da18ba96701df7d367 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ar0-9400000000-2cdebb455e6373988bbc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032664 |
|---|
| FooDB ID | FDB010615 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8058758 |
|---|
| ChEBI ID | 138783 |
|---|
| PubChem Compound ID | 9883083 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|