| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:18:10 UTC |
|---|
| Update Date | 2016-11-09 01:18:21 UTC |
|---|
| Accession Number | CHEM026883 |
|---|
| Identification |
|---|
| Common Name | 1,2,3,4-Tetrahydro-b-carboline-1,3-dicarboxylic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 1,2,3,4-Tetrahydro-b-carboline-1,3-dicarboxylic acid is found in alcoholic beverages. 1,2,3,4-Tetrahydro-b-carboline-1,3-dicarboxylic acid is present in fruit syrups, beer, wines, vinegar and most fermented sauce |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
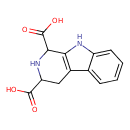
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3,4-Tetrahydro-b-carboline-1,3-dicarboxylate | Generator | | 2,3,4,9-Tetrahydro-1H-pyrido[3,4-b]indole-1,3-dicarboxylic acid, 9ci | HMDB | | 1H,2H,3H,4H,9H-Pyrido[3,4-b]indole-1,3-dicarboxylate | HMDB |
|
|---|
| Chemical Formula | C13H12N2O4 |
|---|
| Average Molecular Mass | 260.245 g/mol |
|---|
| Monoisotopic Mass | 260.080 g/mol |
|---|
| CAS Registry Number | 59132-30-8 |
|---|
| IUPAC Name | 1H,2H,3H,4H,9H-pyrido[3,4-b]indole-1,3-dicarboxylic acid |
|---|
| Traditional Name | 1H,2H,3H,4H,9H-pyrido[3,4-b]indole-1,3-dicarboxylic acid |
|---|
| SMILES | OC(=O)C1CC2=C(NC3=CC=CC=C23)C(N1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C13H12N2O4/c16-12(17)9-5-7-6-3-1-2-4-8(6)14-10(7)11(15-9)13(18)19/h1-4,9,11,14-15H,5H2,(H,16,17)(H,18,19) |
|---|
| InChI Key | ZWMZDKZTZLGVRQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as harmala alkaloids. Harmala alkaloids are compounds with a structure based on harmaline, harmine, harmalol, harman or a derivative of those parents. These parents are beta-carbolines, consisting of a pyrimidine fused to the pyrrole moiety of an indole to form a pyrido[3,4-b]indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Harmala alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Harmala alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Harman
- Beta-carboline
- Pyridoindole
- Alpha-amino acid
- Alpha-amino acid or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- Aralkylamine
- Dicarboxylic acid or derivatives
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014l-2970000000-99c4122806daf841ebd6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000i-6292000000-afc74229239643609ff1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03xr-0090000000-bf5de0622fb3bdf6d4eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-0950000000-03599617ce9d63ab1f93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0159-0910000000-0d1f873d00047a49559a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0aor-0190000000-a1efdde4b26e2bcd8da1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0490000000-79d0bf5e4c201c8e921d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kg-1910000000-7e6b86def5f440df96d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-80cb257510d527557d3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-0960000000-6bb6f823e3b9fe893578 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-1900000000-b15f546013ba18511571 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-90ed7b178f9cf4a275a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03xu-0190000000-dc98942ea22cc2e9c4dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-0900000000-693da81d4516cf05fc39 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032102 |
|---|
| FooDB ID | FDB008820 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 133583 |
|---|
| ChEBI ID | 165178 |
|---|
| PubChem Compound ID | 151564 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|