| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:15:56 UTC |
|---|
| Update Date | 2016-11-09 01:18:20 UTC |
|---|
| Accession Number | CHEM026833 |
|---|
| Identification |
|---|
| Common Name | Gibberellin A110 |
|---|
| Class | Small Molecule |
|---|
| Description | It is used in perfumery and food flavouring. Found in lavender and Ceylon cinnamon oils. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
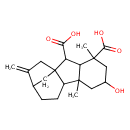
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Linalyl isobutyric acid | Generator | | (1)-1,5-Dimethyl-1-vinylhex-4-enyl isobutyrate | HMDB | | 1,5-Dimethyl-1-vinyl-4-hexenyl 2-methylpropanoate | HMDB | | 1,5-Dimethyl-1-vinyl-4-hexenyl isobutyrate | HMDB | | 1,5-Dimethyl-1-vinylhex-4-enyl isobutyrate | HMDB | | 1,6-Octadien-3-ol, 3,7-dimethyl-, isobutyrate | HMDB | | 1-Ethenyl-1,5-dimethyl-4-hexenyl 2-methylpropanoate | HMDB | | 3, 7-Dimethyl-1,6-octadien-3-yl isobutyrate | HMDB | | 3,7-Dimethyl-1, 6-octadienyl isobutyrate | HMDB | | 3,7-Dimethyl-1,6-octadien-3-ol isobutyrate | HMDB | | 3,7-Dimethyl-1,6-octadien-3-yl 2-methylpropanoate | HMDB | | 3,7-Dimethyl-1,6-octadien-3-yl isobutanoate | HMDB | | 3,7-Dimethyl-1,6-octadien-3-yl isobutyrate | HMDB | | 3,7-Dimethyl-1,6-octadienyl isobutyrate | HMDB | | FEMA 2640 | HMDB | | Isobutyric acid, 1,5-dimethyl-1-vinyl-4-hexenyl ester | HMDB | | Isobutyric acid, linalyl ester | HMDB | | Isobutyric acid, linalyl ester (6ci) | HMDB | | Linalol isobutyrate | HMDB | | Linalool isobutyrate | HMDB | | Linalool, isobutyrate | HMDB | | Linalyl 2-methylpropanoate | HMDB | | GA110 | HMDB | | 6-Hydroxy-4,8-dimethyl-13-methylidenetetracyclo[10.2.1.0¹,⁹.0³,⁸]pentadecane-2,4-dicarboxylate | Generator |
|
|---|
| Chemical Formula | C20H28O5 |
|---|
| Average Molecular Mass | 348.433 g/mol |
|---|
| Monoisotopic Mass | 348.194 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 6-hydroxy-4,8-dimethyl-13-methylidenetetracyclo[10.2.1.0¹,⁹.0³,⁸]pentadecane-2,4-dicarboxylic acid |
|---|
| Traditional Name | 6-hydroxy-4,8-dimethyl-13-methylidenetetracyclo[10.2.1.0¹,⁹.0³,⁸]pentadecane-2,4-dicarboxylic acid |
|---|
| SMILES | CC12CC(O)CC(C)(C1C(C(O)=O)C13CC(CCC21)C(=C)C3)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H28O5/c1-10-6-20-7-11(10)4-5-13(20)18(2)8-12(21)9-19(3,17(24)25)15(18)14(20)16(22)23/h11-15,21H,1,4-9H2,2-3H3,(H,22,23)(H,24,25) |
|---|
| InChI Key | SFGDEUSMQMGAFH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic monoterpenoids. These are monoterpenes that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Acyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic monoterpenoid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0i0r-2379000000-211969b4e05a1ffda520 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0002-1211790000-169d08f8a1b3ceedf342 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0029000000-121779734736a002a1dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gwr-0089000000-964e73e74da5d5f9050b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap0-1591000000-24c0c1f7327f83b285ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0029000000-e32c05fb964f8a65e889 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zg1-0079000000-23e36fb6be0a623c0596 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-2093000000-91856de2536b5101de97 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030426 |
|---|
| FooDB ID | FDB002290 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 6284 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6532 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|