| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:15:36 UTC |
|---|
| Update Date | 2016-11-09 01:18:20 UTC |
|---|
| Accession Number | CHEM026823 |
|---|
| Identification |
|---|
| Common Name | (E)-7-Glucosylzeatin |
|---|
| Class | Small Molecule |
|---|
| Description | Raphanatin is found in root vegetables. Raphanatin is produced by Raphanus sativus (radish |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
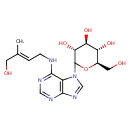
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| e-Zeatin-7-N-glucoside | HMDB | | trans-Zeatin 7-glucoside | HMDB | | trans-Zeatin-7-N-glucoside | HMDB | | 7-beta-D-Glucopyranosylzeatin | HMDB | | Raphanatin | MeSH |
|
|---|
| Chemical Formula | C16H23N5O6 |
|---|
| Average Molecular Mass | 381.384 g/mol |
|---|
| Monoisotopic Mass | 381.165 g/mol |
|---|
| CAS Registry Number | 38165-56-9 |
|---|
| IUPAC Name | (3R,4S,5S,6R)-2-(6-{[(2E)-4-hydroxy-3-methylbut-2-en-1-yl]amino}-7H-purin-7-yl)-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | (3R,4S,5S,6R)-2-(6-{[(2E)-4-hydroxy-3-methylbut-2-en-1-yl]amino}purin-7-yl)-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| SMILES | C\C(CO)=C/CNC1=NC=NC2=C1N(C=N2)C1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C16H23N5O6/c1-8(4-22)2-3-17-14-10-15(19-6-18-14)20-7-21(10)16-13(26)12(25)11(24)9(5-23)27-16/h2,6-7,9,11-13,16,22-26H,3-5H2,1H3,(H,17,18,19)/b8-2+/t9-,11-,12+,13-,16?/m1/s1 |
|---|
| InChI Key | HTDHRCLVWUEXIS-OOHPNJKUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycosylamines. Glycosylamines are compounds consisting of an amine with a beta-N-glycosidic bond to a carbohydrate, thus forming a cyclic hemiaminal ether bond (alpha-amino ether). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glycosylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- N-glycosyl compound
- 6-alkylaminopurine
- 6-aminopurine
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Secondary aliphatic/aromatic amine
- Monosaccharide
- N-substituted imidazole
- Oxane
- Pyrimidine
- Imidolactam
- Azole
- Heteroaromatic compound
- Imidazole
- Secondary alcohol
- Azacycle
- Secondary amine
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Primary alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Alcohol
- Organopnictogen compound
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0mb9-5239000000-5a39e73e49156d9e42bb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-1000-2252169000-191fccb7ee63ec98392d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0230-1079000000-784dc5364c094eff1661 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-3291000000-17692ba35f0617a05bde | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fl9-9660000000-6b52d26cb91702ef6cab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-0189000000-1e8995bf15c5f1606015 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0190000000-0dd95f54e8eb478161d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-1920000000-28bde4aeb05f980ee3d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0093000000-6578557d69d7d9afbcf5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0590000000-17b572eda6befeb0fcbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9i-1960000000-aba701e9a7a98c371cb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0029000000-61ca817869679526309e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-0194000000-0a370e2bd8b930293679 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ue9-0940000000-3b3e96796740552f79bf | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032000 |
|---|
| FooDB ID | FDB008695 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00007549 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013427 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751241 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|