| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:14:30 UTC |
|---|
| Update Date | 2016-11-09 01:18:20 UTC |
|---|
| Accession Number | CHEM026796 |
|---|
| Identification |
|---|
| Common Name | Xanthotoxol arabinoside |
|---|
| Class | Small Molecule |
|---|
| Description | Xanthotoxol arabinoside is found in herbs and spices. Xanthotoxol arabinoside is a constituent of Ruta graveolens (rue) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
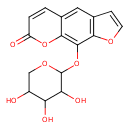
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta,5beta)-3,14-Dihydroxycardo-16,20(22)-dienolide | HMDB | | 3,14-Dihydroxy-(3beta,5beta)-cardo-16,20(22)-dienolide | HMDB | | Rhodexin b | HMDB |
|
|---|
| Chemical Formula | C16H14O8 |
|---|
| Average Molecular Mass | 334.278 g/mol |
|---|
| Monoisotopic Mass | 334.069 g/mol |
|---|
| CAS Registry Number | 160845-06-7 |
|---|
| IUPAC Name | 9-[(3,4,5-trihydroxyoxan-2-yl)oxy]-7H-furo[3,2-g]chromen-7-one |
|---|
| Traditional Name | 9-[(3,4,5-trihydroxyoxan-2-yl)oxy]furo[3,2-g]chromen-7-one |
|---|
| SMILES | OC1COC(OC2=C3OC(=O)C=CC3=CC3=C2OC=C3)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C16H14O8/c17-9-6-22-16(12(20)11(9)19)24-15-13-8(3-4-21-13)5-7-1-2-10(18)23-14(7)15/h1-5,9,11-12,16-17,19-20H,6H2 |
|---|
| InChI Key | HBYXUKYDTXEALZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumarin glycosides. These are aromatic compounds containing a carbohydrate moiety glycosidically bound to a coumarin moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Coumarin glycosides |
|---|
| Direct Parent | Coumarin glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumarin o-glycoside
- Coumarin-8-o-glycoside
- Phenolic glycoside
- Furanocoumarin
- Linear furanocoumarin
- Psoralen
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Benzofuran
- Pyranone
- Benzenoid
- Monosaccharide
- Oxane
- Pyran
- Furan
- Heteroaromatic compound
- Secondary alcohol
- Lactone
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-1000-9467000000-11cc4acf923f323d23c8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-000i-3251390000-6056cd812be7e6acbef0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-0197000000-e91308be05993426e538 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0491000000-4282b36ecaf242d320cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-3790000000-7f0560a31c00b922642e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f89-1189000000-4a0d77e29741bce953f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1492000000-c2f73dc7c9c2d71b77cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2910000000-e205e59c3dfad8b11751 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0091000000-71855bf60108d2d799c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0190000000-a8dcafedd77b31fd446f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1001-5890000000-0482e35bbe4597233225 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0039000000-2fee8eba3c92610db329 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2190000000-16fca9d775ac26d6e96e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0690000000-3b612dbd6ae085bf3779 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031971 |
|---|
| FooDB ID | FDB008663 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057943 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 175254 |
|---|
| PubChem Compound ID | 13846560 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|