| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:12:40 UTC |
|---|
| Update Date | 2016-11-09 01:18:19 UTC |
|---|
| Accession Number | CHEM026744 |
|---|
| Identification |
|---|
| Common Name | (R)-Norisocorydine |
|---|
| Class | Small Molecule |
|---|
| Description | (R)-Norisocorydine is found in fruits. (R)-Norisocorydine is an alkaloid from Annona cherimola (cherimoya |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
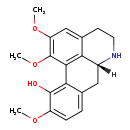
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C19H21NO4 |
|---|
| Average Molecular Mass | 327.374 g/mol |
|---|
| Monoisotopic Mass | 327.147 g/mol |
|---|
| CAS Registry Number | 194923-28-9 |
|---|
| IUPAC Name | (9R)-4,15,16-trimethoxy-10-azatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadeca-1(17),2,4,6,13,15-hexaen-3-ol |
|---|
| Traditional Name | (9R)-4,15,16-trimethoxy-10-azatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadeca-1(17),2,4,6,13,15-hexaen-3-ol |
|---|
| SMILES | [H][C@@]12CC3=CC=C(OC)C(O)=C3C3=C1C(CCN2)=CC(OC)=C3OC |
|---|
| InChI Identifier | InChI=1S/C19H21NO4/c1-22-13-5-4-10-8-12-15-11(6-7-20-12)9-14(23-2)19(24-3)17(15)16(10)18(13)21/h4-5,9,12,20-21H,6-8H2,1-3H3/t12-/m1/s1 |
|---|
| InChI Key | OHDQLTAYHMLRBA-GFCCVEGCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aporphines. These are quinoline alkaloids containing the dibenzo[de,g]quinoline ring system or a dehydrogenated derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aporphines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aporphines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aporphine
- Benzoquinoline
- Phenanthrene
- 1-naphthol
- Naphthalene
- Quinoline
- Tetrahydroisoquinoline
- Anisole
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Azacycle
- Ether
- Secondary aliphatic amine
- Secondary amine
- Organoheterocyclic compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01rt-0094000000-68d559697f2f94079205 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05ai-1019000000-994d45d0dea1d9ba615b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0019000000-76d8dfe1a4fac310d0c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0179000000-c8b0215623f577233630 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01po-0390000000-d59fb94e78b9149c5be5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-924037e1e2fe46925c3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-0029000000-ec4d92da4463cc12473b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gx0-0090000000-dafe5293bb9bf43d9151 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-82e8b7e0c70cdc0f9fe6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0019000000-6bbe7c1b32fe79d86fa3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0091000000-bfb43a8e590b45586ccf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-354160c39518aa1987e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0009000000-354160c39518aa1987e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01r2-0193000000-1475e1e2e4c3a16dd7a1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031921 |
|---|
| FooDB ID | FDB008609 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00026880 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776920 |
|---|
| ChEBI ID | 175179 |
|---|
| PubChem Compound ID | 131751215 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|