| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:10:23 UTC |
|---|
| Update Date | 2016-11-09 01:18:19 UTC |
|---|
| Accession Number | CHEM026687 |
|---|
| Identification |
|---|
| Common Name | Rhapontigenin |
|---|
| Class | Small Molecule |
|---|
| Description | Rhapontigenin is found in garden rhubarb. Rhapontigenin is isolated from rhizomes of Rheum undulatum (rhubarb) 4-Guanidinobutanoate is a normal metabolite present in low concentrations. Patients with hyperargininemia have an arginase deficiency which leads to blockade of the urea cycle in the last step with several clinical symptoms. Owing to the arginase deficiency this patients accumulate arginine which leads eventually to epileptogenic guanidino compounds (PMID 7752905 |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
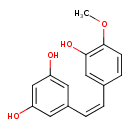
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(3,5-Dihydroxyphenyl)-2-(3-hydroxy-4-methoxyphenyl)ethylene | HMDB | | 3,3',5-Trihydroxy-4'-methoxystilbene | HMDB | | 4'-Methoxy-3,3',5-trihydroxystilbene | HMDB | | 4-Guanidinobutanoic acid | HMDB | | Pontigenin | HMDB |
|
|---|
| Chemical Formula | C15H14O4 |
|---|
| Average Molecular Mass | 258.269 g/mol |
|---|
| Monoisotopic Mass | 258.089 g/mol |
|---|
| CAS Registry Number | 500-65-2 |
|---|
| IUPAC Name | 5-[(Z)-2-(3-hydroxy-4-methoxyphenyl)ethenyl]benzene-1,3-diol |
|---|
| Traditional Name | 5-[(Z)-2-(3-hydroxy-4-methoxyphenyl)ethenyl]benzene-1,3-diol |
|---|
| SMILES | COC1=C(O)C=C(\C=C/C2=CC(O)=CC(O)=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C15H14O4/c1-19-15-5-4-10(8-14(15)18)2-3-11-6-12(16)9-13(17)7-11/h2-9,16-18H,1H3/b3-2- |
|---|
| InChI Key | PHMHDRYYFAYWEG-IHWYPQMZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Resorcinol
- Styrene
- Methoxybenzene
- 1-hydroxy-4-unsubstituted benzenoid
- Phenol
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Ether
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-0490000000-0c7e3c1cc1544fd01557 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0bt9-1001900000-036b5d08a976c1505736 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-cc3e35bd8d646ecf9ddd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0590000000-fb36ae977a4e36814287 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fkl-3920000000-c70ef1d9e39f8d23d409 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-326dbc7596b8abc29f7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-2c9ea4e757e72cb68c00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-2890000000-e1781ac257911d64c7ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-c2a50a42dfada53572a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0190000000-96cc8797cb39e482f4e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-0950000000-a841efaa4f3f27296400 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-5436edd338e0c37ed496 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0960000000-b790cc1b715a020f8232 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g0-0910000000-d0d4c5ff03d7f0ca5185 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031842 |
|---|
| FooDB ID | FDB008524 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00015562 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Rhapontigenin |
|---|
| Chemspider ID | 9055029 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10879760 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Marescau B, De Deyn PP, Holvoet J, Possemiers I, Nagels G, Saxena V, Mahler C: Guanidino compounds in serum and urine of cirrhotic patients. Metabolism. 1995 May;44(5):584-8. | | 2. Jung DB, Lee HJ, Jeong SJ, Lee HJ, Lee EO, Kim YC, Ahn KS, Chen CY, Kim SH: Rhapontigenin inhibited hypoxia inducible factor 1 alpha accumulation and angiogenesis in hypoxic PC-3 prostate cancer cells. Biol Pharm Bull. 2011;34(6):850-5. | | 3. Kim JK, Kim N, Lim YH: Evaluation of the antibacterial activity of rhapontigenin produced from rhapontin by biotransformation against Propionibacterium acnes. J Microbiol Biotechnol. 2010 Jan;20(1):82-7. | | 4. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|