| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:08:24 UTC |
|---|
| Update Date | 2016-11-09 01:18:18 UTC |
|---|
| Accession Number | CHEM026640 |
|---|
| Identification |
|---|
| Common Name | Veranisatin C |
|---|
| Class | Small Molecule |
|---|
| Description | Veranisatin C is found in fruits. Veranisatin C is a constituent of Illicium verum (Chinese star anise) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
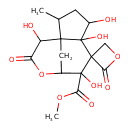
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 4',5',7',11'-tetrahydroxy-2'-methyl-4,10'-dioxo-9'-oxaspiro[oxetane-3,6'-tricyclo[6.3.1.0¹,⁵]dodecane]-7'-carboxylic acid | HMDB | | Veranisatin C | MeSH |
|
|---|
| Chemical Formula | C16H20O10 |
|---|
| Average Molecular Mass | 372.324 g/mol |
|---|
| Monoisotopic Mass | 372.106 g/mol |
|---|
| CAS Registry Number | 182876-51-3 |
|---|
| IUPAC Name | methyl 4',5',7',11'-tetrahydroxy-2'-methyl-4,10'-dioxo-9'-oxaspiro[oxetane-3,6'-tricyclo[6.3.1.0¹,⁵]dodecane]-7'-carboxylate |
|---|
| Traditional Name | methyl 4',5',7',11'-tetrahydroxy-2'-methyl-4,10'-dioxo-9'-oxaspiro[oxetane-3,6'-tricyclo[6.3.1.0¹,⁵]dodecane]-7'-carboxylate |
|---|
| SMILES | COC(=O)C1(O)C2CC3(C(C)CC(O)C3(O)C11COC1=O)C(O)C(=O)O2 |
|---|
| InChI Identifier | InChI=1S/C16H20O10/c1-6-3-7(17)16(23)13(6)4-8(26-10(19)9(13)18)15(22,12(21)24-2)14(16)5-25-11(14)20/h6-9,17-18,22-23H,3-5H2,1-2H3 |
|---|
| InChI Key | JTVRXANWSWLMEV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as terpene lactones. These are prenol lipids containing a lactone ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Terpene lactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Terpene lactone
- Sesquiterpenoid
- Prezizaane sesquiterpenoid
- Tricarboxylic acid or derivatives
- Delta valerolactone
- Delta_valerolactone
- Oxane
- Beta_propiolactone
- Cyclic alcohol
- Methyl ester
- Tertiary alcohol
- Secondary alcohol
- Oxetane
- Lactone
- Carboxylic acid ester
- Polyol
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01r6-4319000000-abc6f61befadd4278005 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0002-2010029000-45a542bb93b8880e740c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05i0-0009000000-bc4612c293edf608cba3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abc-0019000000-7684386e0dce25ea8469 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00r2-1192000000-f017105b49c9516fbaca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0019000000-06ebd6a40c197146be94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00b9-0019000000-daf94b2363aea17042f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pvu-4091000000-765be65d08d644546d6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-924f44af63eba7ce1798 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0091000000-52424c24815291b4d810 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dj-3292000000-18d7176b2e8444ede1cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-1b68dc4328dcad4f3d47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kmr-4079000000-38d75cf56c12da9d8211 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xu-5059000000-7273343d3a8d8d9948a3 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031756 |
|---|
| FooDB ID | FDB008427 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35013380 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 85216671 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|