| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:07:15 UTC |
|---|
| Update Date | 2016-11-09 01:18:18 UTC |
|---|
| Accession Number | CHEM026615 |
|---|
| Identification |
|---|
| Common Name | 1-(Malonylamino)cyclopropanecarboxylic acid |
|---|
| Class | Small Molecule |
|---|
| Description | 1-(Malonylamino)cyclopropanecarboxylic acid is found in cereals and cereal products. 1-(Malonylamino)cyclopropanecarboxylic acid is a constituent of numerous plant species including wheat, tomato and sweet corn |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
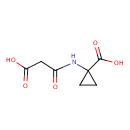
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(Malonylamino)cyclopropanecarboxylate | Generator | | 1-((Carboxyacetyl)amino)-cyclopropanecarboxylic acid | HMDB | | 1-(malonylamino)Cyclopropane-1-carboxylic cid | HMDB | | MACC | HMDB | | Macpca | HMDB | | 1-[(2-Carboxy-1-hydroxyethylidene)amino]cyclopropane-1-carboxylate | Generator |
|
|---|
| Chemical Formula | C7H9NO5 |
|---|
| Average Molecular Mass | 187.150 g/mol |
|---|
| Monoisotopic Mass | 187.048 g/mol |
|---|
| CAS Registry Number | 80550-27-2 |
|---|
| IUPAC Name | 1-(2-carboxyacetamido)cyclopropane-1-carboxylic acid |
|---|
| Traditional Name | 1-(2-carboxyacetamido)cyclopropane-1-carboxylic acid |
|---|
| SMILES | OC(=O)CC(=O)NC1(CC1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H9NO5/c9-4(3-5(10)11)8-7(1-2-7)6(12)13/h1-3H2,(H,8,9)(H,10,11)(H,12,13) |
|---|
| InChI Key | QXOQNNAWFUXKMH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-l-alpha-amino acids. These are n-acylated alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-l-alpha-amino acid
- Cyclopropanecarboxylic acid
- Cyclopropanecarboxylic acid or derivatives
- Dicarboxylic acid or derivatives
- 1,3-dicarbonyl compound
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9400000000-3acd3f362a687b96c53b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-07p3-9340000000-171b3697851c55be046b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uki-1900000000-a4d504f3aa074fe1ef4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-9800000000-dfdddb85c0ea39c0c899 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9100000000-abfb9445811f89561713 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-1900000000-4677195a315c18ca1045 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-6900000000-545f2c85c5757caddca8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zfs-9100000000-2fcdd5ee87d43f4b250f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0019-9700000000-3e14bf9dee8c409e82cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-9000000000-eaba84a42e7c43b9c56d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-0b43fdecf7ba1edd7864 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-9700000000-fb268ddc041401045d33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9200000000-d486b12b5889e2596564 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k96-9000000000-86878074e644050054cb | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031700 |
|---|
| FooDB ID | FDB008363 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 117767 |
|---|
| ChEBI ID | 168168 |
|---|
| PubChem Compound ID | 133503 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|