| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:06:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:18 UTC |
|---|
| Accession Number | CHEM026601 |
|---|
| Identification |
|---|
| Common Name | (9xi,10xi,12xi)-9,10-Dihydroxy-12-octadecenoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | A DiHOME obtained by formal dihydroxylation of the 9,10-double bond of octadeca-9,12-dienoic acid (the 12Z-geoisomer). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
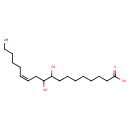
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (12Z)-9,10-Dihydroxyoctadec-12-enoic acid | ChEBI | | 9,10-DHOA | ChEBI | | 9,10-DHOME | ChEBI | | 9,10-Dihydroxy-12Z-octadecenoic acid | ChEBI | | 9,10-Hydroxyoctadec-12(Z)-enoic acid | ChEBI | | Leukotoxin diol | ChEBI | | (12Z)-9,10-Dihydroxyoctadec-12-enoate | Generator | | 9,10-Dihydroxy-12Z-octadecenoate | Generator | | 9,10-Hydroxyoctadec-12(Z)-enoate | Generator | | (9XI,10xi,12xi)-9,10-dihydroxy-12-octadecenoate | Generator | | 9,10-DiHOME | HMDB | | Leukotoxin | HMDB | | Leucotoxins | MeSH, HMDB | | LTX, Actinobacillus actinomycetemcomitans | MeSH, HMDB | | Cytotoxin C (pasteurella haemolytica) | MeSH, HMDB |
|
|---|
| Chemical Formula | C18H34O4 |
|---|
| Average Molecular Mass | 314.460 g/mol |
|---|
| Monoisotopic Mass | 314.246 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (12Z)-9,10-dihydroxyoctadec-12-enoic acid |
|---|
| Traditional Name | 9,10-DiHOME |
|---|
| SMILES | CCCCC\C=C\CC(O)C(O)CCCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H34O4/c1-2-3-4-5-7-10-13-16(19)17(20)14-11-8-6-9-12-15-18(21)22/h7,10,16-17,19-20H,2-6,8-9,11-15H2,1H3,(H,21,22)/b10-7+ |
|---|
| InChI Key | XEBKSQSGNGRGDW-JXMROGBWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Long-chain fatty acid
- Hydroxy fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- 1,2-diol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dm-4920000000-f74ccedf3267a9ce3bf4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-016r-9212320000-21108e48262d95903434 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-03di-0009000000-77cc7fdc656761081db3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-03di-0009000000-ffc80d839bc1257f5b05 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-03di-0009000000-4336a635801eac094dc0 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-03di-0049000000-1d9acdeca87439e56936 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0w29-0194000000-42d4a62f1bd0b85ad15f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0291000000-512176d5b5bef61ddbf1 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0490000000-5c7529e26b7379daa0fc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0890000000-635a557f3e5b47dbbd81 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0umi-0950000000-692dd4f4e4e822232b56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0192000000-bf3198c1147257196565 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fs-8950000000-7afccf770f3ef8a16eda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0537-9200000000-98d69886564adbdc00de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0059000000-437516af25ec795159e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-0952000000-4381456712eb53e26758 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6900000000-121a085ece15cba5122d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031679 |
|---|
| FooDB ID | FDB023415 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8142232 |
|---|
| ChEBI ID | 72663 |
|---|
| PubChem Compound ID | 9966640 |
|---|
| Kegg Compound ID | C14828 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|