| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:06:17 UTC |
|---|
| Update Date | 2016-11-09 01:18:18 UTC |
|---|
| Accession Number | CHEM026590 |
|---|
| Identification |
|---|
| Common Name | Daucic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Daucic acid is found in carrot. Daucic acid is found in wheat, sugar beet and sunflowe |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
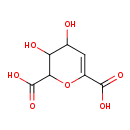
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Daucate | Generator | | (-)-Daucic acid | HMDB | | 2,6-anhydro-3-Deoxy-D-lyxo-hept-2-enaric acid | HMDB | | 2,6-anhydro-3-Deoxy-D-xylo-hept-2-enaric acid, 9ci | HMDB | | 3,4-Dihydroxy-3,4-dihydro-2H-pyran-2,6-dicarboxylate | Generator | | Daucic acid | MeSH |
|
|---|
| Chemical Formula | C7H8O7 |
|---|
| Average Molecular Mass | 204.134 g/mol |
|---|
| Monoisotopic Mass | 204.027 g/mol |
|---|
| CAS Registry Number | 34098-52-7 |
|---|
| IUPAC Name | 3,4-dihydroxy-3,4-dihydro-2H-pyran-2,6-dicarboxylic acid |
|---|
| Traditional Name | 3,4-dihydroxy-3,4-dihydro-2H-pyran-2,6-dicarboxylic acid |
|---|
| SMILES | OC1C=C(OC(C1O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H8O7/c8-2-1-3(6(10)11)14-5(4(2)9)7(12)13/h1-2,4-5,8-9H,(H,10,11)(H,12,13) |
|---|
| InChI Key | KUKCUROTFRBUNU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Pyran
- Dicarboxylic acid or derivatives
- 1,2-diol
- Secondary alcohol
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-c107aeb7f5fd5fa69be9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-016r-6942700000-e3779dfa15819f949fce | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0950000000-71af3f39a1f7a16297c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4u-1910000000-bf7f796da3b777b90dab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-9400000000-6dfd3d6b33643582b672 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-0890000000-b2fa9933f00bfa674b7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-6910000000-608252c11ebb6fcca020 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03xu-9600000000-0f6a1c12bc47c55986b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-0940000000-c4d8344316f2d0a5f49d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-3900000000-0703d2681c2fa32a4d0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100000000-52f45b652b3363968614 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-0900000000-851eb6b2474eb0b654a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05to-6910000000-75879a93910098cfe33a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-9100000000-c411da0ba2fd216bac87 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031665 |
|---|
| FooDB ID | FDB008325 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4475394 |
|---|
| ChEBI ID | 172444 |
|---|
| PubChem Compound ID | 5316316 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|