| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:05:34 UTC |
|---|
| Update Date | 2016-11-09 01:18:17 UTC |
|---|
| Accession Number | CHEM026575 |
|---|
| Identification |
|---|
| Common Name | Orientanol B |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Erythrina glauca (gallito). Orientanol B is found in green vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
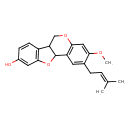
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-O-Methylcalopocarpin | HMDB | | 9-Hydroxy-3-methoxy-2-prenylpterocarpan | HMDB |
|
|---|
| Chemical Formula | C21H22O4 |
|---|
| Average Molecular Mass | 338.397 g/mol |
|---|
| Monoisotopic Mass | 338.152 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-methoxy-4-(3-methylbut-2-en-1-yl)-8,17-dioxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-2(7),3,5,11(16),12,14-hexaen-14-ol |
|---|
| Traditional Name | 5-methoxy-4-(3-methylbut-2-en-1-yl)-8,17-dioxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁶]heptadeca-2(7),3,5,11(16),12,14-hexaen-14-ol |
|---|
| SMILES | COC1=CC2=C(C=C1CC=C(C)C)C1OC3=C(C=CC(O)=C3)C1CO2 |
|---|
| InChI Identifier | InChI=1S/C21H22O4/c1-12(2)4-5-13-8-16-19(10-18(13)23-3)24-11-17-15-7-6-14(22)9-20(15)25-21(16)17/h4,6-10,17,21-22H,5,11H2,1-3H3 |
|---|
| InChI Key | FZFGGNNUYSILSL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pterocarpans. These are benzo-pyrano-furano-benzene compounds, containing the 6H-[1]benzofuro[3,2-c]chromene skeleton. They are derivatives of isoflavonoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Furanoisoflavonoids |
|---|
| Direct Parent | Pterocarpans |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ec-2957000000-9350144d865fbd7621de | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0007-4519000000-a53e7ce1ac89369541e1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-1839000000-9c6dfe0c203a23550a88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ll-3966000000-f5395dda17952c5a5448 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06dl-5900000000-d9d431e36b17251290da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-cc017c470169dbd5c733 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0219000000-11b678c2dc279d38442d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00l2-0911000000-d64de7362f502643388d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0039000000-5628e154efaecfe85550 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-0096000000-2c21db4df135bb57455c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000w-4791000000-8d52211a4a52ccdab626 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-58aebbe019e7e30272d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-73f8911c808a8e945fe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-1196000000-a17f5a61f440d7ff2495 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031633 |
|---|
| FooDB ID | FDB008278 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 1617 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 1680 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|