| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:01:41 UTC |
|---|
| Update Date | 2016-11-09 01:18:17 UTC |
|---|
| Accession Number | CHEM026505 |
|---|
| Identification |
|---|
| Common Name | 8-Hydroxy-6-methoxy-3-methyl-3,4-dihydroisocoumarin |
|---|
| Class | Small Molecule |
|---|
| Description | An isochromane that is mellein bearing a methoxy substituent at position 6. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
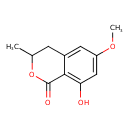
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,4-Dihydro-8-hydroxy-6-methoxy-3-methyl-1H-2-benzopyran-1-one | ChEBI | | 3,4-Dihydro-8-hydroxy-6-methoxy-3-methylisocoumarin | ChEBI | | 3-Methyl-6-methoxy-8-hydroxy-3,4-dihydroisocoumarin | ChEBI | | 6-Methoxy-8-hydroxy-3-methyl-3,4-dihydroisocoumarin | ChEBI | | 6-MHMD-Isocoumarin | ChEBI | | 3,4-dihydro-8-Hydroxy-6-methoxy-3-methyl-1H-2-benzopyran-1-one | HMDB | | Antibiotic LL-N313a | HMDB | | Isocoumarin | HMDB | | LL-N313a | HMDB | | 6-Methoxy-8-hydroxy-3-methyl-3,4-dihydroisocoumarin, (R)-(-)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C11H12O4 |
|---|
| Average Molecular Mass | 208.211 g/mol |
|---|
| Monoisotopic Mass | 208.074 g/mol |
|---|
| CAS Registry Number | 13410-15-6 |
|---|
| IUPAC Name | 8-hydroxy-6-methoxy-3-methyl-3,4-dihydro-1H-2-benzopyran-1-one |
|---|
| Traditional Name | 6-methoxymellein |
|---|
| SMILES | COC1=CC(O)=C2C(=O)OC(C)CC2=C1 |
|---|
| InChI Identifier | InChI=1S/C11H12O4/c1-6-3-7-4-8(14-2)5-9(12)10(7)11(13)15-6/h4-6,12H,3H2,1-2H3 |
|---|
| InChI Key | AIFNAMVERSBWPS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 2-benzopyrans |
|---|
| Direct Parent | 2-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-benzopyran
- Anisole
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Vinylogous acid
- Carboxylic acid ester
- Lactone
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid derivative
- Oxacycle
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03k9-0900000000-061d6423fe204dada398 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dr-1190000000-61e509659cdac5da285d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-5cb43d7f8e2a12058461 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1960000000-d424daf3d806fe39867d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rw-2900000000-6c072322fd99a7553da3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-0890000000-98a8fc6f8d69cf5e11d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bt9-0960000000-acc2bdf7629a8d7b719b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-2900000000-ef5fffbb60c6bd22a8be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-f1b9a89f9653fb07743a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0950000000-552cbacc2fca7862f085 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06g0-2910000000-359bfe42610099dcd1ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-01af25d4eb5cb12278e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bt9-0950000000-ccf50223a1aace736947 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dm-2900000000-129399cd77aecc4086a4 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038510 |
|---|
| FooDB ID | FDB017890 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003004 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 6-Methoxymellein |
|---|
| Chemspider ID | 83993 |
|---|
| ChEBI ID | 16252 |
|---|
| PubChem Compound ID | 93040 |
|---|
| Kegg Compound ID | C02381 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|