| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:57:35 UTC |
|---|
| Update Date | 2016-11-09 01:18:16 UTC |
|---|
| Accession Number | CHEM026389 |
|---|
| Identification |
|---|
| Common Name | Adenosine diphosphate-D-ribose |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
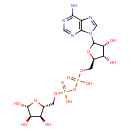
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [({[(2R,3S,4R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]({[(2R,3S,4R,5R)-3,4,5-trihydroxyoxolan-2-yl]methoxy})phosphinate | Generator | | Adenosine diphosphoric acid-D-ribose | Generator |
|
|---|
| Chemical Formula | C15H23N5O14P2 |
|---|
| Average Molecular Mass | 559.316 g/mol |
|---|
| Monoisotopic Mass | 559.072 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[(2R,3S,4R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}({[hydroxy({[(2R,3S,4R,5R)-3,4,5-trihydroxyoxolan-2-yl]methoxy})phosphoryl]oxy})phosphinic acid |
|---|
| Traditional Name | [(2R,3S,4R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy({hydroxy[(2R,3S,4R,5R)-3,4,5-trihydroxyoxolan-2-yl]methoxyphosphoryl}oxy)phosphinic acid |
|---|
| SMILES | NC1=NC=NC2=C1N=CN2C1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@@H](O)[C@H](O)[C@@H]2O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C15H23N5O14P2/c16-12-7-13(18-3-17-12)20(4-19-7)14-10(23)8(21)5(32-14)1-30-35(26,27)34-36(28,29)31-2-6-9(22)11(24)15(25)33-6/h3-6,8-11,14-15,21-25H,1-2H2,(H,26,27)(H,28,29)(H2,16,17,18)/t5-,6-,8-,9-,10-,11-,14?,15-/m1/s1 |
|---|
| InChI Key | SRNWOUGRCWSEMX-MRUDJCSFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as purine nucleotide sugars. These are purine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleotides |
|---|
| Sub Class | Purine nucleotide sugars |
|---|
| Direct Parent | Purine nucleotide sugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine nucleotide sugar
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Pyrimidine
- Alkyl phosphate
- Imidolactam
- Phosphoric acid ester
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Hemiacetal
- Organoheterocyclic compound
- Polyol
- Azacycle
- Oxacycle
- Organic oxide
- Amine
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Hydrocarbon derivative
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0910130000-b9073b1bbcee0d4c7c6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0910000000-4407a7719b31b18bb57b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1900000000-9d6c2b567f9da9d1d1ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a59-1701390000-e958d4521e35989750e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1910100000-a653cbe7cba6b5fab234 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a7i-3900000000-c8a10ccf06e1cb8b548a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0102090000-d6f3e5e258d1e2fde2f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7a-0956630000-06d72e7c1baf65bb1bcf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1921000000-252dd33ec57f82d79e9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-6ee1df1c137698a2098a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1210590000-f67396d292bd0392dc42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054k-9742500000-fb8808153fd7853f46d2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303049 |
|---|
| FooDB ID | FDB007502 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59696436 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 146879505 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|