| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:57:09 UTC |
|---|
| Update Date | 2016-11-09 01:18:15 UTC |
|---|
| Accession Number | CHEM026376 |
|---|
| Identification |
|---|
| Common Name | Leptosine |
|---|
| Class | Small Molecule |
|---|
| Description | Leptosine, also known as leptosin j, is a member of the class of compounds known as pyrroloindoles. Pyrroloindoles are compounds containing a pyrroloindole moiety, which is a tricyclic heterocycle which consists of a pyrrole ring fused to an indole. Pyrrole is 5-membered ring consisting of four carbon atoms and one nitrogen atom. Indole is a bicyclic compound consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Leptosine is slightly soluble (in water) and a very weakly acidic compound (based on its pKa). Leptosine can be found in american cranberry, which makes leptosine a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
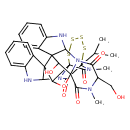
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Leptosin I | MeSH | | Leptosin J | MeSH |
|
|---|
| Chemical Formula | C32H32N6O7S4 |
|---|
| Average Molecular Mass | 740.892 g/mol |
|---|
| Monoisotopic Mass | 740.122 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 37-hydroxy-7-(hydroxymethyl)-6,36-dimethyl-30-(propan-2-yl)-3-oxa-31,32,33,34-tetrathia-6,9,11,26,28,36-hexaazadecacyclo[28.4.2.1⁴,¹⁸.0¹,²⁸.0²,¹⁹.0⁴,⁹.0¹⁰,¹⁸.0¹²,¹⁷.0¹⁹,²⁷.0²⁰,²⁵]heptatriaconta-12,14,16,20,22,24-hexaene-5,8,29,35-tetrone |
|---|
| Traditional Name | 37-hydroxy-7-(hydroxymethyl)-30-isopropyl-6,36-dimethyl-3-oxa-31,32,33,34-tetrathia-6,9,11,26,28,36-hexaazadecacyclo[28.4.2.1⁴,¹⁸.0¹,²⁸.0²,¹⁹.0⁴,⁹.0¹⁰,¹⁸.0¹²,¹⁷.0¹⁹,²⁷.0²⁰,²⁵]heptatriaconta-12,14,16,20,22,24-hexaene-5,8,29,35-tetrone |
|---|
| SMILES | CC(C)C12SSSSC3(C4OC56C(O)C7(C(NC8=CC=CC=C78)N5C(=O)C(CO)N(C)C6=O)C44C(NC5=CC=CC=C45)N3C1=O)C(=O)N2C |
|---|
| InChI Identifier | InChI=1S/C32H32N6O7S4/c1-14(2)31-27(44)38-24-29(16-10-6-8-12-18(16)34-24)22(32(38,26(43)36(31)4)47-49-48-46-31)45-30-21(41)28(29)15-9-5-7-11-17(15)33-23(28)37(30)20(40)19(13-39)35(3)25(30)42/h5-12,14,19,21-24,33-34,39,41H,13H2,1-4H3 |
|---|
| InChI Key | PZFMMBJJDMZAIP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrroloindoles. Pyrroloindoles are compounds containing a pyrroloindole moiety, which is a tricyclic heterocycle which consists of a pyrrole ring fused to an indole. Pyrrole is 5-membered ring consisting of four carbon atoms and one nitrogen atom. Indole is a bicyclic compound consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Pyrroloindoles |
|---|
| Direct Parent | Pyrroloindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrroloindole
- Alpha-amino acid or derivatives
- Indole
- Dihydroindole
- Thiodioxopiperazine
- Dioxopiperazine
- 2,5-dioxopiperazine
- Meta-oxazepine
- Secondary aliphatic/aromatic amine
- N-alkylpiperazine
- N-methylpiperazine
- 1,4-diazinane
- Oxane
- Piperazine
- Benzenoid
- Pyrrolidine
- Pyrrole
- Tertiary carboxylic acid amide
- Secondary alcohol
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Oxacycle
- Secondary amine
- Azacycle
- Carboxylic acid derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0010000900-c082d9e8b0560ab50050 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000100700-c94aa9ce92390139fbf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-9110000100-b2e06acdcdd000fe8bb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4r-0010001900-c0f934de68ed85ab2f7b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-3100109200-d62cf78ef7f9ec46a8ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-3062594000-a34f2ba151e07b9955c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000000900-98ee2d25bdf48a82d34e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0000000900-767790758bf9fd7d8bb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0000000900-306693bfa1bc0111c050 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000900-53b02b5b7cb6707d0957 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-0000001900-bfb8f2e61dea8521e825 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-6000006900-8e0eb52a6575905244c6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB007445 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 157801 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|