| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:54:42 UTC |
|---|
| Update Date | 2016-11-09 01:18:15 UTC |
|---|
| Accession Number | CHEM026299 |
|---|
| Identification |
|---|
| Common Name | cis-Violaxanthin |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
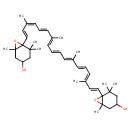
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C40H56O4 |
|---|
| Average Molecular Mass | 600.870 g/mol |
|---|
| Monoisotopic Mass | 600.418 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 6-[(1E,3Z,5E,7E,9E,11E,13E,15E,17E)-18-{4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl}-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]-1,5,5-trimethyl-7-oxabicyclo[4.1.0]heptan-3-ol |
|---|
| Traditional Name | 6-[(1E,3Z,5E,7E,9E,11E,13E,15E,17E)-18-{4-hydroxy-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptan-1-yl}-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]-1,5,5-trimethyl-7-oxabicyclo[4.1.0]heptan-3-ol |
|---|
| SMILES | C/C(/C=C/C=C(/C)\C=C\C12OC1(C)CC(O)CC2(C)C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C12OC1(C)CC(O)CC2(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56O4/c1-29(17-13-19-31(3)21-23-39-35(5,6)25-33(41)27-37(39,9)43-39)15-11-12-16-30(2)18-14-20-32(4)22-24-40-36(7,8)26-34(42)28-38(40,10)44-40/h11-24,33-34,41-42H,25-28H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,29-15+,30-16+,31-19-,32-20+ |
|---|
| InChI Key | SZCBXWMUOPQSOX-VPYIVGMTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Oxepane
- Cyclic alcohol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Ether
- Oxirane
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0032597000-af64159ae48b8e1fa899 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02ai-0295531000-02d9985c675cc61fb2a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03xr-3494200000-f5d8d58d659ed07feca7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-ced1b1342da183c34f3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0100090000-01c9d49ff3b710660a47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-3700490000-fec974be88afdb5602d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0011092000-616fb8bff0a6bcc74ce7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0012290000-b312a11edc7cd7432753 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0171-2239430000-8af32ab5bfa67570b0bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0300090000-43c86f6f93850f3afb14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0101290000-7bc5d7e172bc297a22ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0315190000-efe6c7c2ed7a99c422ba | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302970 |
|---|
| FooDB ID | FDB007098 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4946510 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6442428 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|