| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:51:57 UTC |
|---|
| Update Date | 2016-11-09 01:18:14 UTC |
|---|
| Accession Number | CHEM026222 |
|---|
| Identification |
|---|
| Common Name | 1,6-di-O-Galloylglucose |
|---|
| Class | Small Molecule |
|---|
| Description | A galloyl-beta-D-glucose compound having two galloyl groups in the 1- and 6-positions. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
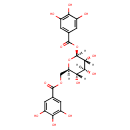
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-O,6-O-Digalloyl-beta-D-glucose | ChEBI | | 1-O,6-O-Digalloyl-b-D-glucose | Generator | | 1-O,6-O-Digalloyl-β-D-glucose | Generator | | 1,6-Bis-O-galloyl-b-D-glucose | Generator | | 1,6-Bis-O-galloyl-β-D-glucose | Generator | | DGG16 CPD | MeSH | | 1,6-Di-O-galloyl-beta-D-glucose | MeSH |
|
|---|
| Chemical Formula | C20H20O14 |
|---|
| Average Molecular Mass | 484.366 g/mol |
|---|
| Monoisotopic Mass | 484.085 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | [(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(3,4,5-trihydroxybenzoyloxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | 1,6-bis-O-galloyl-β-D-glucose |
|---|
| SMILES | [H][C@]1(COC(=O)C2=CC(O)=C(O)C(O)=C2)O[C@@]([H])(OC(=O)C2=CC(O)=C(O)C(O)=C2)[C@]([H])(O)[C@@]([H])(O)[C@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C20H20O14/c21-8-1-6(2-9(22)13(8)25)18(30)32-5-12-15(27)16(28)17(29)20(33-12)34-19(31)7-3-10(23)14(26)11(24)4-7/h1-4,12,15-17,20-29H,5H2/t12-,15-,16+,17-,20+/m1/s1 |
|---|
| InChI Key | LYGRISUQIZNHGM-IVABAYMNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tannins. These are naturally occurring polyphenols which be categorized into four main classes: hydrolyzable tannin (based on ellagic acid or gallic acid), condensed tannins (made of oligomeric or polymeric proanthocyanidins), complex tannins (made of a catechin bound to a gallotannin or elagitannin), and phlorotannins (oligomers of phloroglucinol). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Tannins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tannins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tannin
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Benzoate ester
- Benzenetriol
- Benzoic acid or derivatives
- Pyrogallol derivative
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Monosaccharide
- Benzenoid
- Oxane
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Secondary alcohol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Polyol
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gbi-0917800000-c457dceb1da2a4c0f60d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0912100000-8947c60a980f8633b051 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1900000000-cdf97965c27b0edf9ae7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0159-0912500000-2bd15c1295338c2b9310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0901000000-db11dd1164818be5d87e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1900000000-807e365640c3b6686539 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fri-0811900000-56da5f863f4901ec73d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uy1-0961400000-db62cdce054f149194d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-0920100000-5d7ff3ece2d689eae6dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0101900000-acafc1f5b1e7da58a29b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-0938400000-6cbcfa5ad46f2dbb604b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-0900100000-227165175cf258eb778c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302910 |
|---|
| FooDB ID | FDB006838 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 389206 |
|---|
| ChEBI ID | 15723 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C04101 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|