| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:51:31 UTC |
|---|
| Update Date | 2016-11-09 01:18:13 UTC |
|---|
| Accession Number | CHEM026209 |
|---|
| Identification |
|---|
| Common Name | Catechin-(4->8)-gallocatechin |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
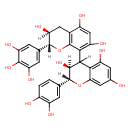
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C30H26O13 |
|---|
| Average Molecular Mass | 594.520 g/mol |
|---|
| Monoisotopic Mass | 594.137 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2R,3S)-8-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol |
|---|
| Traditional Name | (2R,3S)-8-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol |
|---|
| SMILES | O[C@H]1CC2=C(O[C@@H]1C1=CC(O)=C(O)C(O)=C1)C(C1[C@H](O)[C@H](OC3=C1C(O)=CC(O)=C3)C1=CC=C(O)C(O)=C1)=C(O)C=C2O |
|---|
| InChI Identifier | InChI=1S/C30H26O13/c31-12-6-17(35)23-22(7-12)42-29(10-1-2-14(32)16(34)3-10)27(41)25(23)24-18(36)9-15(33)13-8-21(39)28(43-30(13)24)11-4-19(37)26(40)20(38)5-11/h1-7,9,21,25,27-29,31-41H,8H2/t21-,25?,27-,28+,29+/m0/s1 |
|---|
| InChI Key | YJMNEZANCYQLJR-JNJHKLINSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biflavonoids and polyflavonoids. These are organic compounds containing at least two flavan/flavone units. These units are usually linked through CC or C-O-C bonds. Some examples include C2-O-C3, C2-O-C4, C3'-C3''', and C6-C8''. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Biflavonoids and polyflavonoids |
|---|
| Direct Parent | Biflavonoids and polyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - B-type proanthocyanidin
- Bi- and polyflavonoid skeleton
- Proanthocyanidin
- Epigallocatechin
- Catechin
- 3'-hydroxyflavonoid
- Flavan-3-ol
- Hydroxyflavonoid
- 3-hydroxyflavonoid
- 7-hydroxyflavonoid
- 5-hydroxyflavonoid
- 4'-hydroxyflavonoid
- Flavan
- Chromane
- 1-benzopyran
- Benzopyran
- Benzenetriol
- Pyrogallol derivative
- Catechol
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Ether
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0100290000-bd4f18c46f020cd99da2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-0912120000-6d32e43a05b4bebd71e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0910000000-105a7c53098878a04181 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000090000-a3a584fd6c175c79d042 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-0100590000-4f3b18c998b884781bc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002r-0301290000-53fb971c5297dd514456 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0211890000-d5b650a85ab7f45eacd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0655920000-b914f11a8d347fec26a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0553-0791110000-4c30768e361a06215d52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000190000-63964720cf6c3557dfa0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0101790000-ec891b4a82efcadbbc3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0109-0602490000-062b7cc109aaba0d2eb6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302900 |
|---|
| FooDB ID | FDB006771 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59696414 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|