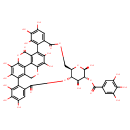| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:51:29 UTC |
|---|
| Update Date | 2016-11-09 01:18:13 UTC |
|---|
| Accession Number | CHEM026208 |
|---|
| Identification |
|---|
| Common Name | 2-O-Galloyl-(4S,6S)-gallagoyl-D-glucose |
|---|
| Class | Small Molecule |
|---|
| Description | 2-o-galloyl-(4s,6s)-gallagoyl-d-glucose is slightly soluble (in water) and a very weakly acidic compound (based on its pKa). 2-o-galloyl-(4s,6s)-gallagoyl-d-glucose can be found in pomegranate, which makes 2-o-galloyl-(4s,6s)-gallagoyl-d-glucose a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C41H28O25 |
|---|
| Average Molecular Mass | 920.646 g/mol |
|---|
| Monoisotopic Mass | 920.092 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (10S,11S,12R,13R,15R)-3,4,5,11,13,21,22,23,26,27,38,39-dodecahydroxy-8,18,35-trioxo-9,14,17,29,36-pentaoxaoctacyclo[29.8.0.0²,⁷.0¹⁰,¹⁵.0¹⁹,²⁴.0²⁵,³⁴.0²⁸,³³.0³²,³⁷]nonatriaconta-1(31),2,4,6,19,21,23,25,27,32(37),33,38-dodecaen-12-yl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | (10S,11S,12R,13R,15R)-3,4,5,11,13,21,22,23,26,27,38,39-dodecahydroxy-8,18,35-trioxo-9,14,17,29,36-pentaoxaoctacyclo[29.8.0.0²,⁷.0¹⁰,¹⁵.0¹⁹,²⁴.0²⁵,³⁴.0²⁸,³³.0³²,³⁷]nonatriaconta-1(31),2,4,6,19,21,23,25,27,32(37),33,38-dodecaen-12-yl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | O[C@@H]1O[C@@H]2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C4OCC5=C(C(O)=C(O)C6=C5C4=C3C(=O)O6)C3=C(O)C(O)=C(O)C=C3C(=O)O[C@H]2[C@H](O)[C@H]1OC(=O)C1=CC(O)=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C41H28O25/c42-11-1-7(2-12(43)23(11)46)37(56)66-36-32(55)33-15(63-41(36)60)6-62-38(57)8-3-13(44)25(48)27(50)17(8)20-22-21-19-10(5-61-34(21)30(53)29(20)52)18(28(51)31(54)35(19)65-40(22)59)16-9(39(58)64-33)4-14(45)24(47)26(16)49/h1-4,15,32-33,36,41-55,60H,5-6H2/t15-,32+,33-,36-,41-/m1/s1 |
|---|
| InChI Key | JMCHOJWVWCHHFC-MUAAPLJWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pterocarpans. These are benzo-pyrano-furano-benzene compounds, containing the 6H-[1]benzofuro[3,2-c]chromene skeleton. They are derivatives of isoflavonoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Furanoisoflavonoids |
|---|
| Direct Parent | Pterocarpans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoflavanol
- Pterocarpan
- Isoflavan
- Chromane
- Benzopyran
- 1-benzopyran
- Benzofuran
- Coumaran
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Ether
- Organoheterocyclic compound
- Oxacycle
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0611061519-888392d4c04759cb47da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900071655-02c7a942c98d68e42f32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w29-0800390000-daf3d507c05f03c767a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-0810005597-ce63210c9477387e7a92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1910000111-04a1480e382398342272 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-1900001000-717053c94736b70a3bc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0200000009-e81c4274ee763fb99cd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gb9-0910003323-c5a5d3873972815b6da0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fr-3800006980-92d477a9eacd6590caae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000009-3fd6d93fc80c8b8c2382 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0500000349-743f6a7f7d75e6cdeb7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1920004441-8ec02c64282521cc90ef | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB006760 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|