| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:49:05 UTC |
|---|
| Update Date | 2016-11-09 01:18:13 UTC |
|---|
| Accession Number | CHEM026141 |
|---|
| Identification |
|---|
| Common Name | Phaseic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
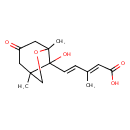
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E)-5-{8-hydroxy-1,5-dimethyl-3-oxo-6-oxabicyclo[3.2.1]octan-8-yl}-3-methylpenta-2,4-dienoate | Generator | | Phaseate | Generator |
|
|---|
| Chemical Formula | C15H20O5 |
|---|
| Average Molecular Mass | 280.316 g/mol |
|---|
| Monoisotopic Mass | 280.131 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2E,4E)-5-{8-hydroxy-1,5-dimethyl-3-oxo-6-oxabicyclo[3.2.1]octan-8-yl}-3-methylpenta-2,4-dienoic acid |
|---|
| Traditional Name | (2E,4E)-5-{8-hydroxy-1,5-dimethyl-3-oxo-6-oxabicyclo[3.2.1]octan-8-yl}-3-methylpenta-2,4-dienoic acid |
|---|
| SMILES | C\C(\C=C\C1(O)C2(C)COC1(C)CC(=O)C2)=C/C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H20O5/c1-10(6-12(17)18)4-5-15(19)13(2)7-11(16)8-14(15,3)20-9-13/h4-6,19H,7-9H2,1-3H3,(H,17,18)/b5-4+,10-6+ |
|---|
| InChI Key | IZGYIFFQBZWOLJ-UMCKCUICSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as abscisic acids and derivatives. These are terpene compounds containing the abscisic acid moiety, which is characterized by a 3-methylpenta-2,4-dienoic acid attached to the C1 carbon of a 4-oxocyclohex-2-ene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Abscisic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Abscisic acid
- Medium-chain fatty acid
- Branched fatty acid
- Heterocyclic fatty acid
- Hydroxy fatty acid
- Oxepane
- Methyl-branched fatty acid
- Fatty acyl
- Fatty acid
- Unsaturated fatty acid
- Cyclic alcohol
- Tertiary alcohol
- Tetrahydrofuran
- Ketone
- Cyclic ketone
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Ether
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0090000000-6835c7113bf5ceb20a10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01bi-0190000000-156b07330945b2ad4b81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-7970000000-4f12dc78488206aae6ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004r-0190000000-d21fdf7a899c368d10bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00p0-1190000000-6da619f64cddd28f9026 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053j-3790000000-c3ababeb0eb3646f396a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-029t-0090000000-01bbc923a22af0a41ef9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4j-1690000000-467f08066a949b8b4f3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015i-7490000000-d79c05ce7852488a12a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-364143652fa826f28f8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0170-0390000000-a4a8a7b72cc53fd956e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gdi-2590000000-102ac2d7508972472d17 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302844 |
|---|
| FooDB ID | FDB006507 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4516055 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|