| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:48:45 UTC |
|---|
| Update Date | 2016-11-09 01:18:13 UTC |
|---|
| Accession Number | CHEM026133 |
|---|
| Identification |
|---|
| Common Name | Oryzanol B |
|---|
| Class | Small Molecule |
|---|
| Description | Oryzanol b is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Oryzanol b can be found in rice, which makes oryzanol b a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
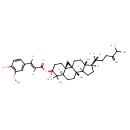
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| gamma-Oryzanol | MeSH | | Triacontane 3-(4-hydroxy-3-methoxyphenyl)-2-propenate | MeSH | | Oryzanol | MeSH |
|
|---|
| Chemical Formula | C41H60O4 |
|---|
| Average Molecular Mass | 616.927 g/mol |
|---|
| Monoisotopic Mass | 616.449 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1S,3R,6S,8R,11S,15R,16R)-7,7,11,16-tetramethyl-15-[(2R)-6-methyl-5-methylideneheptan-2-yl]pentacyclo[9.7.0.0¹,³.0³,⁸.0¹²,¹⁶]octadecan-6-yl (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate |
|---|
| Traditional Name | (1S,3R,6S,8R,11S,15R,16R)-7,7,11,16-tetramethyl-15-[(2R)-6-methyl-5-methylideneheptan-2-yl]pentacyclo[9.7.0.0¹,³.0³,⁸.0¹²,¹⁶]octadecan-6-yl (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate |
|---|
| SMILES | [H]\C(=C(\[H])C1=CC(OC)=C(O)C=C1)C(=O)O[C@@]1([H])CC[C@]23C[C@]22CC[C@]4(C)[C@]([H])(CCC4([H])[C@]2(C)CC[C@@]3([H])C1(C)C)[C@]([H])(C)CCC(=C)C(C)C |
|---|
| InChI Identifier | InChI=1S/C41H60O4/c1-26(2)27(3)10-11-28(4)30-14-16-34-38(30,7)22-23-41-25-40(41)21-19-35(37(5,6)33(40)18-20-39(34,41)8)45-36(43)17-13-29-12-15-31(42)32(24-29)44-9/h12-13,15,17,24,26,28,30,33-35,42H,3,10-11,14,16,18-23,25H2,1-2,4-9H3/b17-13+/t28-,30-,33+,34?,35+,38-,39+,40-,41+/m1/s1 |
|---|
| InChI Key | ZIWPYEIAPMTNTE-WTHHACQLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Steroid ester
- Cinnamic acid or derivatives
- Coumaric acid or derivatives
- Hydroxycinnamic acid or derivatives
- Cinnamic acid ester
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Styrene
- Methoxybenzene
- Phenol
- Alkyl aryl ether
- Fatty acid ester
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Fatty acyl
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Ether
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-016r-1700759000-c836d8eb058087868e29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0059-4904621000-bbf4d9dd579210f122ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-5319311000-b8ecb74dd6c45d44bc9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0200509000-f99d6d664ee5ba8b1190 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0600912000-af990366e6122d5b5ba1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00g0-1400900000-9f8e345c823b189d07a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01dj-0029425000-08fb367e891704958cbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-067j-9501011000-f04ca9876508726a5505 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lr-9400000000-a375cae9338f80c42092 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000309000-d01e52ae601e25b0b7d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ds-0900545000-b4ca086a8314993c9904 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00au-1900170000-8b1c779dad480ff084c9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB006413 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 24868322 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|