| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:47:13 UTC |
|---|
| Update Date | 2016-11-09 01:18:12 UTC |
|---|
| Accession Number | CHEM026095 |
|---|
| Identification |
|---|
| Common Name | (+)-Cycloolivil |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
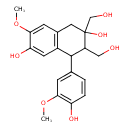
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C20H24O7 |
|---|
| Average Molecular Mass | 376.400 g/mol |
|---|
| Monoisotopic Mass | 376.152 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4-(4-hydroxy-3-methoxyphenyl)-2,3-bis(hydroxymethyl)-7-methoxy-1,2,3,4-tetrahydronaphthalene-2,6-diol |
|---|
| Traditional Name | 4-(4-hydroxy-3-methoxyphenyl)-2,3-bis(hydroxymethyl)-7-methoxy-3,4-dihydro-1H-naphthalene-2,6-diol |
|---|
| SMILES | COC1=CC2=C(C=C1O)C(C(CO)C(O)(CO)C2)C1=CC(OC)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C20H24O7/c1-26-17-5-11(3-4-15(17)23)19-13-7-16(24)18(27-2)6-12(13)8-20(25,10-22)14(19)9-21/h3-7,14,19,21-25H,8-10H2,1-2H3 |
|---|
| InChI Key | KCIQZCNOUZCRGH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 9,9p-dihydroxyaryltetralin lignans. These are lignans with a structure based on the 1-phenyltetralin skeleton carrying a hydroxyl group at the 9- and the 9'- position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Aryltetralin lignans |
|---|
| Sub Class | 9,9p-dihydroxyaryltetralin lignans |
|---|
| Direct Parent | 9,9p-dihydroxyaryltetralin lignans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 9,9p-dihydroxyaryltetralin lignan
- Tetralin
- Methoxyphenol
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Tertiary alcohol
- Ether
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0009000000-9ac059f6ce5f5d883b95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6u-0229000000-74a37d1933ec27e70751 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002o-0396000000-c76cdd97bbd72b5ae5e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-6e5c91d1f8e8cb9b19ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-8412b0d081ed1addba3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0019000000-aea3b35702f930a894c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-27ed87d6a6bef07309ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-0169000000-159981c7ec0077435923 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-009i-0359000000-17cae166f1d3b4feb721 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-fb278c5e7bfbad20c65e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-71ff23ff361fc9f243c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0009000000-ad91dea4164cd8355d69 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302798 |
|---|
| FooDB ID | FDB006299 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 205220 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|