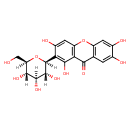| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:44:23 UTC |
|---|
| Update Date | 2016-11-09 01:18:11 UTC |
|---|
| Accession Number | CHEM026015 |
|---|
| Identification |
|---|
| Common Name | Mangiferol |
|---|
| Class | Small Molecule |
|---|
| Description | A C-glycosyl compound consisting of 1,3,6,7-tetrahydroxyxanthen-9-one having a beta-D-glucosyl residue at the 6-position. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1S)-1,5-Anhydro-1-(1,3,6,7-tetrahydroxy-9-oxo-9H-xanthen-2-yl)-D-glucitol | ChEBI | | Alpizarin | ChEBI | | Aphloiol | ChEBI | | Chinomin | ChEBI | | Chinonin | ChEBI | | Hedysarid | ChEBI | | 2-beta-D-Glucopyranosyl-1,3,6,7-tetrahydroxy-9H-xanthen-9-one | MeSH |
|
|---|
| Chemical Formula | C19H18O11 |
|---|
| Average Molecular Mass | 422.342 g/mol |
|---|
| Monoisotopic Mass | 422.085 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 1,3,6,7-tetrahydroxy-2-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]-9H-xanthen-9-one |
|---|
| Traditional Name | mangiferin |
|---|
| SMILES | [H][C@]1(CO)O[C@@]([H])(C2=C(O)C3=C(OC4=C(C=C(O)C(O)=C4)C3=O)C=C2O)[C@]([H])(O)[C@@]([H])(O)[C@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C19H18O11/c20-4-11-15(25)17(27)18(28)19(30-11)12-8(23)3-10-13(16(12)26)14(24)5-1-6(21)7(22)2-9(5)29-10/h1-3,11,15,17-23,25-28H,4H2/t11-,15-,17+,18-,19+/m1/s1 |
|---|
| InChI Key | AEDDIBAIWPIIBD-ZJKJAXBQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthones. These are polycyclic aromatic compounds containing a xanthene moiety conjugated to a ketone group at carbon 9. Xanthene is a tricyclic compound made up of two benzene rings linearly fused to each other through a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Xanthones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- Xanthone
- Hexose monosaccharide
- C-glycosyl compound
- Chromone
- Glycosyl compound
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Pyranone
- Benzenoid
- Pyran
- Oxane
- Monosaccharide
- Heteroaromatic compound
- Vinylogous acid
- Secondary alcohol
- Oxacycle
- Polyol
- Dialkyl ether
- Ether
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Primary alcohol
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0001900000-cd181f4b807807e7b271 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-5444900000-859d9e4fab35209339b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05tr-3191000000-38d081044a4db9297e57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1012900000-7ebb421bc67cffd995f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kmi-9356600000-a6bc5b543a306d155a96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9362000000-7748484710203ff0e957 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-24777d788daa0f49e535 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0091100000-a605617618423baaacea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-4291000000-8175993e0388775d7c41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0012900000-099c672f233cb722473d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0q2i-0059200000-dc339254058bf57c31b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-5393000000-4428606d67dafb97210a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302730 |
|---|
| FooDB ID | FDB005974 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002962 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Mangiferin |
|---|
| Chemspider ID | 4444966 |
|---|
| ChEBI ID | 6682 |
|---|
| PubChem Compound ID | 5281647 |
|---|
| Kegg Compound ID | C10077 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|