| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:43:44 UTC |
|---|
| Update Date | 2016-11-09 01:18:11 UTC |
|---|
| Accession Number | CHEM026000 |
|---|
| Identification |
|---|
| Common Name | beta-Chamigrene |
|---|
| Class | Small Molecule |
|---|
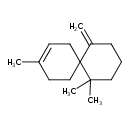
| Description | A carbobicyclic compound and sesquiterpene that is spiro[5.5]undec-2-ene which is substituted by a methylidene group at position 11 and by methyl groups at positions 3, 7 and 7. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-Chamigrene | Generator | | Β-chamigrene | Generator | | (S)-Isochamigrene | MeSH | | Chamigrene | MeSH | | Isochamigrene | MeSH |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3,7,7-trimethyl-11-methylidenespiro[5.5]undec-2-ene |
|---|
| Traditional Name | β-chamigrene |
|---|
| SMILES | CC1=CCC2(CC1)C(=C)CCCC2(C)C |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-12-7-10-15(11-8-12)13(2)6-5-9-14(15,3)4/h7H,2,5-6,8-11H2,1,3-4H3 |
|---|
| InChI Key | WLNGPDPILFYWKF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chamigranes. These are sesquiterpenoids characterized by a 1,1,5,9-tetramethylspiro[5,5]undecane skeleton, formally obtained by linking the C1-C6 and C6-C11 of farnesane together. They are predominantly isolated from algae. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Chamigranes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chamigrane sesquiterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1390000000-abb55af960fe631eb394 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-5920000000-ec816ab398dec7756e43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0j4r-7900000000-857b09b117205c443b05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-48e732fd8f0cbce7aaa3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-f76a7363e71b7bda6bf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1910000000-b6c52ddc600dc5a74696 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-4980000000-56f15a9b6005ed8834b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053i-8910000000-5247d2ce35ab0532f831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-37263daab0322d802418 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0090000000-8c28a556bc5a47f43e10 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302718 |
|---|
| FooDB ID | FDB005917 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003116 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 27047 |
|---|
| ChEBI ID | 61744 |
|---|
| PubChem Compound ID | 29073 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|