| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:43:35 UTC |
|---|
| Update Date | 2016-11-09 01:18:11 UTC |
|---|
| Accession Number | CHEM025995 |
|---|
| Identification |
|---|
| Common Name | Kaempferol 3-rutinosyl 7-O-alpha-L-rhamnoside |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
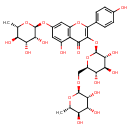
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Kaempferol 3-rutinosyl 7-O-a-L-rhamnoside | Generator | | Kaempferol 3-rutinosyl 7-O-α-L-rhamnoside | Generator |
|
|---|
| Chemical Formula | C33H40O19 |
|---|
| Average Molecular Mass | 740.659 g/mol |
|---|
| Monoisotopic Mass | 740.216 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-hydroxy-2-(4-hydroxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-7-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-chromen-4-one |
|---|
| Traditional Name | 5-hydroxy-2-(4-hydroxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-7-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}chromen-4-one |
|---|
| SMILES | C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](OC3=C(OC4=C(C(O)=CC(O[C@@H]5O[C@@H](C)[C@H](O)[C@@H](O)[C@H]5O)=C4)C3=O)C3=CC=C(O)C=C3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C33H40O19/c1-10-19(36)23(40)26(43)31(47-10)46-9-17-21(38)25(42)28(45)33(51-17)52-30-22(39)18-15(35)7-14(49-32-27(44)24(41)20(37)11(2)48-32)8-16(18)50-29(30)12-3-5-13(34)6-4-12/h3-8,10-11,17,19-21,23-28,31-38,40-45H,9H2,1-2H3/t10-,11-,17+,19-,20-,21+,23+,24+,25-,26+,27+,28+,31+,32-,33-/m0/s1 |
|---|
| InChI Key | PEFASEPMJYRQBW-DQNJWZGDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chamigranes. These are sesquiterpenoids characterized by a 1,1,5,9-tetramethylspiro[5,5]undecane skeleton, formally obtained by linking the C1-C6 and C6-C11 of farnesane together. They are predominantly isolated from algae. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Chamigranes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chamigrane sesquiterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00wm-0140890700-2c4eb9eba9862ac83a1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-0190640000-db3533fb6c5cb1ed9b5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0290210000-8299d15ef6a5564aec0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-3414564900-9d01ec59f914d436b69c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03na-4953871400-0799865ba43f3bd19cab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001r-2493400000-0f134e38a29a5c5d4aa0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000900200-996ab5f48cf12018467e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000x-0000900900-e1324d510b24aaa4cfe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0000900000-fa0a1381502338409394 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000900-f0058e4130bf776e99d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-0000500900-2ec4b4ddb6316e3d0fc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0000900000-ac239bcf92abd2356870 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302713 |
|---|
| FooDB ID | FDB005906 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10290057 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|