| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:43:26 UTC |
|---|
| Update Date | 2016-11-09 01:18:11 UTC |
|---|
| Accession Number | CHEM025991 |
|---|
| Identification |
|---|
| Common Name | Santamarin |
|---|
| Class | Small Molecule |
|---|
| Description | A sesquiterpene lactone of the eudesmanolide group. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
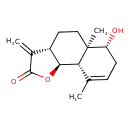
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Santamarine | ChEBI | | (3AS)-2,3,3abeta,4,5,5a,6,7,9abeta,9balpha-decahydro-6alpha-hydroxy-5aalpha,9-dimethyl-3-methylenenaphtho[1,2-b]furan-2-one | ChEBI | | (3AS,5ar,6R,9as,9BS)-6-hydroxy-5a,9-dimethyl-3-methylene-3a,4,5,5a,6,7,9a,9b-octahydro-3H-naphtho[1,2-b]furan-2-one | ChEBI | | (3AS,5ar,6R,9as,9BS)-6-hydroxy-5a,9-dimethyl-3-methylidene-4,5,6,7,9a,9b-hexahydro-3ah-benzo[g][1]benzofuran-2-one | ChEBI | | Balchanin | ChEBI | | Santamarine | ChEBI | | (3AS)-2,3,3abeta,4,5,5a,6,7,9abeta,9balpha-decahydro-6a-hydroxy-5aalpha,9-dimethyl-3-methylenenaphtho[1,2-b]furan-2-one | Generator | | (3AS)-2,3,3abeta,4,5,5a,6,7,9abeta,9balpha-decahydro-6α-hydroxy-5aalpha,9-dimethyl-3-methylenenaphtho[1,2-b]furan-2-one | Generator |
|
|---|
| Chemical Formula | C15H20O3 |
|---|
| Average Molecular Mass | 248.318 g/mol |
|---|
| Monoisotopic Mass | 248.141 g/mol |
|---|
| CAS Registry Number | 4290-13-5 |
|---|
| IUPAC Name | (3aS,5aR,6R,9aS,9bS)-6-hydroxy-5a,9-dimethyl-3-methylidene-2H,3H,3aH,4H,5H,5aH,6H,7H,9aH,9bH-naphtho[1,2-b]furan-2-one |
|---|
| Traditional Name | (+)-santamarine |
|---|
| SMILES | CC1=CC[C@@H](O)[C@]2(C)CC[C@@H]3[C@H](OC(=O)C3=C)[C@@H]12 |
|---|
| InChI Identifier | InChI=1S/C15H20O3/c1-8-4-5-11(16)15(3)7-6-10-9(2)14(17)18-13(10)12(8)15/h4,10-13,16H,2,5-7H2,1,3H3/t10-,11+,12+,13-,15-/m0/s1 |
|---|
| InChI Key | PLSSEPIRACGCBO-PFFFPCNUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eudesmanolides, secoeudesmanolides, and derivatives. These are terpenoids with a structure based on the eudesmanolide (a 3,5a,9-trimethyl-naphtho[1,2-b]furan-2-one derivative) or secoeudesmanolide (a 3,6-dimethyl-5-(pentan-2-yl)-1-benzofuran-2-one derivative) skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Eudesmanolides, secoeudesmanolides, and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Eudesmanolide
- Sesquiterpenoid
- Naphthofuran
- Gamma butyrolactone
- Tetrahydrofuran
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Secondary alcohol
- Lactone
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0290000000-789eefe074e1b12c084d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f8a-1940000000-dc7583a16431bb675821 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1003-9310000000-6677ba80414870d17185 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-d29ad4e4535fa5263291 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0190000000-997de3e007cc13186660 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-3920000000-2246bdfb040620ee52eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-b55c1d7eb34f2d130aca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0390000000-aa9891c63c3267405779 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-1910000000-296693f45a2dbc58341a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-7b3a58b796a0e4263959 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-7e0e38a32353911a24e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016u-3390000000-b3cad309313dc9047892 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302709 |
|---|
| FooDB ID | FDB005897 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003363 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 163664 |
|---|
| ChEBI ID | 9023 |
|---|
| PubChem Compound ID | 188297 |
|---|
| Kegg Compound ID | C09544 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|