| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:41:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:10 UTC |
|---|
| Accession Number | CHEM025928 |
|---|
| Identification |
|---|
| Common Name | ent-Kauran-16-beta-ol |
|---|
| Class | Small Molecule |
|---|
| Description | Ent-kauran-16-beta-ol is a member of the class of compounds known as kaurane diterpenoids. Kaurane diterpenoids are diterpene alkaloids with a structure that is based on the kaurane skeleton. Kaurane is a tetracyclic compound that arises by cyclisation of a pimarane precursor followed by rearrangement. It possesses a [3,2,1]-bicyclic ring system with C15-C16 bridge connected to C13, forming the five-membered ring D. Ent-kauran-16-beta-ol is practically insoluble (in water) and an extremely weak acidic compound (based on its pKa). Ent-kauran-16-beta-ol can be found in sunflower, which makes ent-kauran-16-beta-ol a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
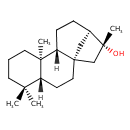
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| ent-Kauran-16-b-ol | Generator | | ent-Kauran-16-β-ol | Generator |
|
|---|
| Chemical Formula | C20H34O |
|---|
| Average Molecular Mass | 290.483 g/mol |
|---|
| Monoisotopic Mass | 290.261 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1S,4R,9R,10R,13R,14R)-5,5,9,14-tetramethyltetracyclo[11.2.1.0¹,¹⁰.0⁴,⁹]hexadecan-14-ol |
|---|
| Traditional Name | (1S,4R,9R,10R,13R,14R)-5,5,9,14-tetramethyltetracyclo[11.2.1.0¹,¹⁰.0⁴,⁹]hexadecan-14-ol |
|---|
| SMILES | [H][C@@]12CC[C@@H]3C[C@]1(C[C@@]3(C)O)CC[C@]1([H])C(C)(C)CCC[C@@]21C |
|---|
| InChI Identifier | InChI=1S/C20H34O/c1-17(2)9-5-10-18(3)15(17)8-11-20-12-14(6-7-16(18)20)19(4,21)13-20/h14-16,21H,5-13H2,1-4H3/t14-,15-,16+,18-,19-,20+/m1/s1 |
|---|
| InChI Key | FZSRMADKTOBCNT-HFJXXIIPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as kaurane diterpenoids. These are diterpene alkaloids with a structure that is based on the kaurane skeleton. Kaurane is a tetracyclic compound that arises by cyclisation of a pimarane precursor followed by rearrangement. It possesses a [3,2,1]-bicyclic ring system with C15-C16 bridge connected to C13, forming the five-membered ring D. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Kaurane diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Kaurane diterpenoid
- Tertiary alcohol
- Cyclic alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0090000000-330e59126f493c30893c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-1190000000-2c9fcabc0367fb3d2a8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-4590000000-eb2395023f401bdf04dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-bc4fdccb38655679ebdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-299155b6520deef1aa63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-0090000000-23d6e772738faaeb3bd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-81df27efb721f4dbaa63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00du-2960000000-3333a4207d35af637894 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ei-6920000000-0eb62017d248b3f8348d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e782630883d1b4130674 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-e782630883d1b4130674 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0090000000-d300e9e82a53533000b4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302663 |
|---|
| FooDB ID | FDB005671 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59696374 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 21593607 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|