| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:37:50 UTC |
|---|
| Update Date | 2016-11-09 01:18:09 UTC |
|---|
| Accession Number | CHEM025833 |
|---|
| Identification |
|---|
| Common Name | Apigenin 7-glucuronosyl-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
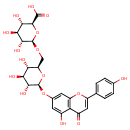
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-{[(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C27H28O16 |
|---|
| Average Molecular Mass | 608.502 g/mol |
|---|
| Monoisotopic Mass | 608.138 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-3,4,5-trihydroxy-6-{[(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6R)-3,4,5-trihydroxy-6-{[(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-{[5-hydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid |
|---|
| SMILES | O[C@H]1[C@H](O)[C@@H](CO[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)O[C@@H](OC2=CC3=C(C(O)=C2)C(=O)C=C(O3)C2=CC=C(O)C=C2)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C27H28O16/c28-10-3-1-9(2-4-10)14-7-13(30)17-12(29)5-11(6-15(17)41-14)40-27-23(36)19(32)18(31)16(42-27)8-39-26-22(35)20(33)21(34)24(43-26)25(37)38/h1-7,16,18-24,26-29,31-36H,8H2,(H,37,38)/t16-,18-,19+,20+,21+,22-,23-,24+,26-,27-/m1/s1 |
|---|
| InChI Key | ADUXEKGZLYFWEG-VQFZQRBBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-7-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C7-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-7-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavonoid-7-o-glycoside
- Hydroxyflavonoid
- Flavone
- 5-hydroxyflavonoid
- 4'-hydroxyflavonoid
- Phenolic glycoside
- O-glucuronide
- 1-o-glucuronide
- Glucuronic acid or derivatives
- Chromone
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Beta-hydroxy acid
- 1-hydroxy-4-unsubstituted benzenoid
- Pyranone
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Hydroxy acid
- Pyran
- Oxane
- Vinylogous acid
- Heteroaromatic compound
- Secondary alcohol
- Acetal
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0190162000-1484abf718630af2e3e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0190100000-010d252b8bc46ed2d9fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0290000000-aa87c268e4b9c1a325a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-066r-3691366000-bd6429d5e0e96e55d998 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2590020000-dda67283000ab9971a04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2390000000-cf17a1af6a2c0c85a3eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000109000-9947bcb5d33853c796b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0api-0050709000-7373df2f085559191caa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0090001000-dae41b11a12fdc1d1e75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0090004000-33523e86035f70589b55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00f0-0090901000-6c3616754d5335b91747 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0090000000-60b322ff74af8a765580 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302596 |
|---|
| FooDB ID | FDB005299 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59696356 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|