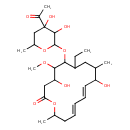| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:37:47 UTC |
|---|
| Update Date | 2016-11-09 01:18:09 UTC |
|---|
| Accession Number | CHEM025831 |
|---|
| Identification |
|---|
| Common Name | PL |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| DDAXHPP-II | MeSH | | 5-O-(4',6'-Dideoxy-3'-C-acetyl-beta-D-xylohexopyranosyl)platenolide-II | MeSH |
|
|---|
| Chemical Formula | C28H46O10 |
|---|
| Average Molecular Mass | 542.659 g/mol |
|---|
| Monoisotopic Mass | 542.309 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (11E,13E)-6-[(4-acetyl-3,4-dihydroxy-6-methyloxan-2-yl)oxy]-7-ethyl-4,10-dihydroxy-5-methoxy-9,16-dimethyl-1-oxacyclohexadeca-11,13-dien-2-one |
|---|
| Traditional Name | (11E,13E)-6-[(4-acetyl-3,4-dihydroxy-6-methyloxan-2-yl)oxy]-7-ethyl-4,10-dihydroxy-5-methoxy-9,16-dimethyl-1-oxacyclohexadeca-11,13-dien-2-one |
|---|
| SMILES | CCC1CC(C)C(O)\C=C\C=C\CC(C)OC(=O)CC(O)C(OC)C1OC1OC(C)CC(O)(C1O)C(C)=O |
|---|
| InChI Identifier | InChI=1S/C28H46O10/c1-7-20-13-16(2)21(30)12-10-8-9-11-17(3)36-23(32)14-22(31)25(35-6)24(20)38-27-26(33)28(34,19(5)29)15-18(4)37-27/h8-10,12,16-18,20-22,24-27,30-31,33-34H,7,11,13-15H2,1-6H3/b9-8+,12-10+ |
|---|
| InChI Key | IZFPDEQYDFAJRK-CDKJVOIVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as macrolides and analogues. These are organic compounds containing a lactone ring of at least twelve members. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolides and analogues |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Macrolides and analogues |
|---|
| Alternative Parents | |
|---|
| Substituents | - Macrolide
- Oxane
- Alpha-hydroxy ketone
- Tertiary alcohol
- Secondary alcohol
- Carboxylic acid ester
- Lactone
- Ketone
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Ether
- Dialkyl ether
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uml-0009170000-36e3614c78cdb0252949 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-2119000000-ace259c5f6eb6878110b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfu-9112000000-1c038046ba83fafd8e3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2606690000-712e6a9dc29e9f4d9047 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxu-5309320000-6a81d4b7423225f0ae16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mp-9135000000-6ccd4e4f439f51f237bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056u-0103390000-0e122b89ef3410a0f7b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0309050000-3c8d1d808de984b6cbef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0019-1109000000-9020a973801f2330805a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0002490000-3dddb06ca304cb63830e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0904140000-f34fd98ff7ca26053c60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fe0-1609020000-0f1e503a3f1b6032810e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302594 |
|---|
| FooDB ID | FDB005293 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4532249 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5385029 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|