| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:34:40 UTC |
|---|
| Update Date | 2016-11-09 01:18:08 UTC |
|---|
| Accession Number | CHEM025741 |
|---|
| Identification |
|---|
| Common Name | (Z,E)-alpha-Farnesene |
|---|
| Class | Small Molecule |
|---|
| Description | The (Z,E)-stereoiosmer of alpha-farnesene. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
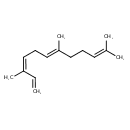
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3Z,6E)-a-Farnesene | Generator | | (3Z,6E)-Α-farnesene | Generator | | (Z,E)-a-Farnesene | Generator | | (Z,E)-α-Farnesene | Generator | | (3Z,6E)-3,7,11-Trimethyl-1,3,6,10-dodecatetraene | HMDB | | (3Z,6E)-alpha-Farnesene | HMDB | | (Z,E)-alpha-Farnesene | HMDB | | 3,7,11-Trimethyl-1,3,6,10-dodecatetraene | HMDB | | Farnesene | HMDB | | Zataroside A | HMDB | | alpha-Farnesene | HMDB | | cis,trans-alpha-Farnesene | HMDB | | cis,trans-α-Farnesene | HMDB |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (3Z,6E)-3,7,11-trimethyldodeca-1,3,6,10-tetraene |
|---|
| Traditional Name | (Z,E)-α-farnesene |
|---|
| SMILES | CC(C)=CCC\C(C)=C\C\C=C(\C)C=C |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-6-14(4)10-8-12-15(5)11-7-9-13(2)3/h6,9-10,12H,1,7-8,11H2,2-5H3/b14-10-,15-12+ |
|---|
| InChI Key | CXENHBSYCFFKJS-OXYODPPFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Farsesane sesquiterpenoid
- Sesquiterpenoid
- Alkatetraene
- Branched unsaturated hydrocarbon
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Acyclic olefin
- Hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-3690000000-313b913d532cd4decec1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pw9-9710000000-078e5a8d7e85822b7c0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-9100000000-3d3f85f913cafb612d5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-6d4cdcd69849df029e45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-e363a25bab51e774a592 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kg9-4900000000-b4cda282a594b52f2539 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-060s-7910000000-05be940f3488abb12fe8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a7l-9300000000-a54209dd2c1271d1d7d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05ru-9000000000-a7b8e3b259690a16a441 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0190000000-fa0c676eabe0c49e322b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0390000000-0d353a60a5a97cd9c1a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-4900000000-9f5bab6c0c653c72230e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036066 |
|---|
| FooDB ID | FDB004888 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00035167 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-9338 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Farnesene |
|---|
| Chemspider ID | 4515350 |
|---|
| ChEBI ID | 39238 |
|---|
| PubChem Compound ID | 5362889 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|