| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:34:10 UTC |
|---|
| Update Date | 2016-11-09 01:18:08 UTC |
|---|
| Accession Number | CHEM025728 |
|---|
| Identification |
|---|
| Common Name | beta-Cubebene |
|---|
| Class | Small Molecule |
|---|
| Description | Beta-cubebene, also known as (-)-B-cubebene, is a member of the class of compounds known as sesquiterpenoids. Sesquiterpenoids are terpenes with three consecutive isoprene units. Beta-cubebene is a citrus and fruity tasting compound and can be found in a number of food items such as sweet basil, roman camomile, pot marjoram, and sweet bay, which makes beta-cubebene a potential biomarker for the consumption of these food products. Beta-cubebene can be found primarily in saliva. Piper cubeba, cubeb or tailed pepper is a plant in genus Piper, cultivated for its fruit and essential oil. It is mostly grown in Java and Sumatra, hence sometimes called Java pepper. The fruits are gathered before they are ripe, and carefully dried. Commercial cubebs consist of the dried berries, similar in appearance to black pepper, but with stalks attached – the "tails" in "tailed pepper". The dried pericarp is wrinkled, and its color ranges from grayish brown to black. The seed is hard, white and oily. The odor of cubebs is described as agreeable and aromatic and the taste as pungent, acrid, slightly bitter and persistent. It has been described as tasting like allspice, or like a cross between allspice and black pepper . |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
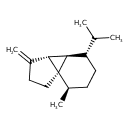
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-beta-Cubebene | ChEBI | | (3AS-(3aalpha,3bbata,4beta,7alpha,7as*))-octahydro-7-methyl-3-methylene-4-(1-methylethyl)-1Hcyclopenta(1,3)cyclopropa(1,2)benzene | ChEBI | | (-)-b-Cubebene | Generator | | (-)-Β-cubebene | Generator | | (3AS-(3aalpha,3bbata,4b,7a,7as*))-octahydro-7-methyl-3-methylene-4-(1-methylethyl)-1Hcyclopenta(1,3)cyclopropa(1,2)benzene | Generator | | (3AS-(3aalpha,3bbata,4β,7α,7as*))-octahydro-7-methyl-3-methylene-4-(1-methylethyl)-1Hcyclopenta(1,3)cyclopropa(1,2)benzene | Generator | | b-Cubebene | Generator | | Β-cubebene | Generator | | (3AS-(3aalpha,3bbata,4b,7a,7aa*))-octahydro-7-methyl-3-methylene-4-(1-methylethyl)-1Hcyclopenta(1,3)cyclopropa(1,2)benzene | HMDB | | (3AS-(3aalpha,3bbata,4S,7R,7as*))-octahydro-7-methyl-3-methylene-4-(1-methylethyl)-1H-cyclopenta(1,3)cyclopropa(1,2)benzene | HMDB |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | 13744-15-5 |
|---|
| IUPAC Name | (1R,5S,6R,7S,10R)-10-methyl-4-methylidene-7-(propan-2-yl)tricyclo[4.4.0.0¹,⁵]decane |
|---|
| Traditional Name | (-)-β-cubebene |
|---|
| SMILES | CC(C)[C@@H]1CC[C@@H](C)[C@@]23CCC(=C)[C@@H]2[C@@H]13 |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-9(2)12-6-5-11(4)15-8-7-10(3)13(15)14(12)15/h9,11-14H,3,5-8H2,1-2,4H3/t11-,12+,13-,14-,15+/m1/s1 |
|---|
| InChI Key | FSRZGYRCMPZNJF-KHMAMNHCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01rm-2900000000-dbacb878626acfeae4ab | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1390000000-6a944b8e46ab50de2220 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-5940000000-af588cea8e1fb3c0a9d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pxr-9500000000-f65be015faf4afb50b4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7e9c1e42af69f59fea70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-79206d02912ecd5353d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1910000000-9155330998fee11bfb19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-5590000000-3e03bee8035f33df3400 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9350000000-d48bf4c96f38a991dc20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-0dac7d29bf1b8eb1d6a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0061853 |
|---|
| FooDB ID | FDB004832 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003121 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-8803 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Piper cubeba |
|---|
| Chemspider ID | 84031 |
|---|
| ChEBI ID | 10363 |
|---|
| PubChem Compound ID | 93081 |
|---|
| Kegg Compound ID | C09648 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Debrauwere J, Verzele M: Constituents of peppers: IV. The hydrocarbons of pepper essential oil. J Chromatogr Sci. 1976 Jun;14(6):296-8. | | 2. Kasali AA, Ekundayo O, Paul C, Konig WA: epi-Cubebanes from Solidago canadensis. Phytochemistry. 2002 Apr;59(8):805-10. | | 3. Sajjadi SE, Ghannadi A, Sajjadi SE, Ghannadi A: Volatile oil composition of the aerial parts of Ajuga orientalis L. from Iran. Z Naturforsch C. 2004 Mar-Apr;59(3-4):166-8. | | 4. Nickavar B, Amin G, Yosefi M: Volatile constituents of the flower and fruit oils of Pittosporum tobira (Thunb.) Ait. grown in Iran. Z Naturforsch C. 2004 Mar-Apr;59(3-4):174-6. | | 5. Skalicka-Wozniak K, Ludwiczuk A, Widelski J, Filipe JJ, Asakawa Y, Glowniak K: Volatile constituents of Ocimum minimum herb cultivated in Portugal. Nat Prod Commun. 2009 Oct;4(10):1383-6. | | 6. Park SY, Park SJ, Park NJ, Joo WH, Lee SJ, Choi YW: alpha-Iso-cubebene exerts neuroprotective effects in amyloid beta stimulated microglia activation. Neurosci Lett. 2013 Oct 25;555:143-8. doi: 10.1016/j.neulet.2013.09.053. Epub 2013 Sep 30. | | 7. Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. | | 8. Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. | | 9. Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. | | 10. Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. | | 11. The lipid handbook with CD-ROM | | 12. Wikipedia: http://en.wikipedia.org/wiki/Beta-cubebene_synthase | | 13. MetaCyc: beta-cubebene biosynthesis: http://metacyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6290 | | 14. UniProt B3TPQ6 : Beta-cubebene synthase: http://www.uniprot.org/uniprot/B3TPQ6 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=12587209 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=15241917 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=19370937 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=19449812 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=20401796 | | 20. https://www.ncbi.nlm.nih.gov/pubmed/?term=20433088 |
|
|---|