| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:30:43 UTC |
|---|
| Update Date | 2016-11-09 01:18:07 UTC |
|---|
| Accession Number | CHEM025623 |
|---|
| Identification |
|---|
| Common Name | Loliolide |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
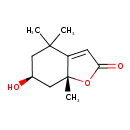
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3-Dihydroxy-3,5,5-trimethylcyclohexylidene-4-acetic acid lactone | MeSH | | (3S5R)-Loliolide | ChEMBL | | (-)-Loliolide | PhytoBank | | Calendin | PhytoBank | | Caulilide | PhytoBank | | Digiprolactone | PhytoBank | | Loliolid | PhytoBank |
|
|---|
| Chemical Formula | C11H16O3 |
|---|
| Average Molecular Mass | 196.243 g/mol |
|---|
| Monoisotopic Mass | 196.110 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (6S,7aR)-6-hydroxy-4,4,7a-trimethyl-2,4,5,6,7,7a-hexahydro-1-benzofuran-2-one |
|---|
| Traditional Name | (6S,7aR)-6-hydroxy-4,4,7a-trimethyl-6,7-dihydro-5H-1-benzofuran-2-one |
|---|
| SMILES | C[C@@]12C[C@@H](O)CC(C)(C)C1=CC(=O)O2 |
|---|
| InChI Identifier | InChI=1S/C11H16O3/c1-10(2)5-7(12)6-11(3)8(10)4-9(13)14-11/h4,7,12H,5-6H2,1-3H3/t7-,11+/m0/s1 |
|---|
| InChI Key | XEVQXKKKAVVSMW-WRWORJQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzofurans. These are organic compounds containing a benzene ring fused to a furan. Furan is a five-membered aromatic ring with four carbon atoms and one oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzofuran
- 2-furanone
- Cyclic alcohol
- Dihydrofuran
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Lactone
- Secondary alcohol
- Oxacycle
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Alcohol
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0900000000-6f306b0310ea7c7af9db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-2900000000-5ff620161c23c5cf12b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9300000000-2a23cc4430c83159676c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-6108e76a31fa2da2edec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0900000000-7340bdb68c651f84d7f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udj-4900000000-8adc3d43afd5361af707 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-282fa0a3896fa29cd168 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kcs-6900000000-e67a4aa467d8a8de4071 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l5-9200000000-f10742d565af9290493c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-be98c24425dfaa2cd53f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016s-0900000000-de74d71950fc1d3cc9a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-0900000000-c1072f488fcf77a9fefb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302428 |
|---|
| FooDB ID | FDB004536 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019144 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 90666 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 100332 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|