| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:29:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:07 UTC |
|---|
| Accession Number | CHEM025584 |
|---|
| Identification |
|---|
| Common Name | (24S)-24-Ethylbrassinone |
|---|
| Class | Small Molecule |
|---|
| Description | (24s)-24-ethylbrassinone is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). (24s)-24-ethylbrassinone can be found in tea, which makes (24s)-24-ethylbrassinone a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
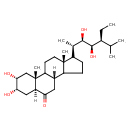
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C29H50O5 |
|---|
| Average Molecular Mass | 478.704 g/mol |
|---|
| Monoisotopic Mass | 478.366 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2R,4R,5S,7S,10S,15S)-14-[(3R,4R,5S)-5-ethyl-3,4-dihydroxy-6-methylheptan-2-yl]-4,5-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-8-one |
|---|
| Traditional Name | (2R,4R,5S,7S,10S,15S)-14-[(3R,4R,5S)-5-ethyl-3,4-dihydroxy-6-methylheptan-2-yl]-4,5-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-8-one |
|---|
| SMILES | [H][C@@]12CC(=O)[C@@]3([H])C[C@H](O)[C@H](O)C[C@]3(C)C1CC[C@]1(C)C(CCC21)C(C)[C@@H](O)[C@H](O)[C@@H](CC)C(C)C |
|---|
| InChI Identifier | InChI=1S/C29H50O5/c1-7-17(15(2)3)27(34)26(33)16(4)19-8-9-20-18-12-23(30)22-13-24(31)25(32)14-29(22,6)21(18)10-11-28(19,20)5/h15-22,24-27,31-34H,7-14H2,1-6H3/t16?,17-,18-,19?,20?,21?,22+,24-,25+,26+,27+,28+,29+/m0/s1 |
|---|
| InChI Key | WADMTJKRYLAHQV-CVNRPTOBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stigmastanes and derivatives. These are sterol lipids with a structure based on the stigmastane skeleton, which consists of a cholestane moiety bearing an ethyl group at the carbon atom C24. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Stigmastanes and derivatives |
|---|
| Direct Parent | Stigmastanes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - C24-propyl-sterol-skeleton
- Triterpenoid
- Stigmastane-skeleton
- Tetrahydroxy bile acid, alcohol, or derivatives
- Ecdysteroid
- Hydroxy bile acid, alcohol, or derivatives
- 23-hydroxysteroid
- Bile acid, alcohol, or derivatives
- 22-hydroxysteroid
- 3-hydroxysteroid
- 6-oxosteroid
- 3-alpha-hydroxysteroid
- Oxosteroid
- 2-hydroxysteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0002900000-1d1a29074e8400f75db2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-029e-7209600000-f309eb3405ef437741a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014j-9306300000-71af89c1445a96e8a321 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0001900000-2dd22e3132594313f5e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057r-4308900000-52223610856d38fe6c86 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02a9-9307300000-3a2debfe0c2014cc180a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00pl-1004900000-541df60b3c3346f76ca7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-6019100000-15b5d656f5b0a93e5cd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9411000000-9571d7b7452340475516 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-82a6b58788334f89bd8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9204700000-de4948a37233fc307004 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-2002900000-37836d39038d491cee85 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302393 |
|---|
| FooDB ID | FDB004405 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 157009875 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|