| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:26:07 UTC |
|---|
| Update Date | 2016-11-09 01:18:06 UTC |
|---|
| Accession Number | CHEM025499 |
|---|
| Identification |
|---|
| Common Name | Haematin |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
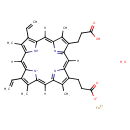
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| -iron(2+) ion 10-(2-carboxyethyl)-14-(2-carboxylatoethyl)-5,20-diethenyl-4,9,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1,.1,.1,]tetracosa-1,3,5,7,9,11(23),12,14,16(22),17,19-undecaen-21-ide hydric acid | Generator | | Λ²-iron(2+) ion 10-(2-carboxyethyl)-14-(2-carboxylatoethyl)-5,20-diethenyl-4,9,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16(22),17,19-undecaen-21-ide hydric acid | Generator |
|
|---|
| Chemical Formula | C34H34FeN4O5 |
|---|
| Average Molecular Mass | 634.514 g/mol |
|---|
| Monoisotopic Mass | 634.188 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | λ²-iron(2+) ion 10-(2-carboxyethyl)-14-(2-carboxylatoethyl)-5,20-diethenyl-4,9,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16(22),17,19-undecaen-21-ide hydrate |
|---|
| Traditional Name | λ²-iron(2+) ion 10-(2-carboxyethyl)-14-(2-carboxylatoethyl)-5,20-diethenyl-4,9,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16(22),17,19-undecaen-21-ide hydrate |
|---|
| SMILES | O.[Fe++].[H]\C-1=C2\[N-]\C(=C([H])/C3=N/C(=C([H])\C4=N\C(=C([H])/C5=C(C=C)C(C)=C-1N5)\C(C)=C4CCC(O)=O)/C(CCC([O-])=O)=C3C)C(C)=C2C=C |
|---|
| InChI Identifier | InChI=1S/C34H34N4O4.Fe.H2O/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);;1H2/q;+2;/p-2/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16-;; |
|---|
| InChI Key | CODDGSIFXYHHJO-HXFTUNQESA-L |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|