| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:25:56 UTC |
|---|
| Update Date | 2016-11-09 01:18:06 UTC |
|---|
| Accession Number | CHEM025495 |
|---|
| Identification |
|---|
| Common Name | Kaempferol 3-O-beta-D-galactopyranoside |
|---|
| Class | Small Molecule |
|---|
| Description | A beta-D-galactoside compound with a 4',5,7-trihydroxychromen-3-yl group at the anomeric position. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
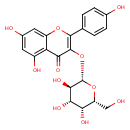
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Trifolioside | ChEBI | | Kaempferol 3-O-beta-D-galactoside | Kegg | | Kaempferol 3-O-b-D-galactoside | Generator | | Kaempferol 3-O-β-D-galactoside | Generator | | Kaempferol 3-O-b-D-galactopyranoside | HMDB | | Kaempferol 3-O-β-D-galactopyranoside | HMDB | | Kaempferol-3-O-galactoside | HMDB | | Kaempferol 3-galactoside | HMDB | | Kaempferol 3-O-D-galactoside | HMDB | | Kaempferol 3-O-galactopyranoside | HMDB | | Kaempferol 3-O-galactoside | HMDB | | Kaempferol-3-O-D-galactopyranoside | HMDB | | Kaempferol 3-O-beta-D-galactopyranoside | HMDB | | Kaempferol 3-O-beta-galactoside | HMDB | | Kaempferol 3-O-β-galactoside | HMDB | | Kaempferol 3-beta-D-galactopyranoside | HMDB | | Kaempferol 3-β-D-galactopyranoside | HMDB | | Kaempferol 3-beta-D-galactoside | HMDB | | Kaempferol 3-β-D-galactoside | HMDB | | Trifoliin | HMDB | | Trifolin | ChEBI |
|
|---|
| Chemical Formula | C21H20O11 |
|---|
| Average Molecular Mass | 448.377 g/mol |
|---|
| Monoisotopic Mass | 448.101 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-chromen-4-one |
|---|
| Traditional Name | trifolin |
|---|
| SMILES | OC[C@H]1O[C@@H](OC2=C(OC3=C(C(O)=CC(O)=C3)C2=O)C2=CC=C(O)C=C2)[C@H](O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H20O11/c22-7-13-15(26)17(28)18(29)21(31-13)32-20-16(27)14-11(25)5-10(24)6-12(14)30-19(20)8-1-3-9(23)4-2-8/h1-6,13,15,17-18,21-26,28-29H,7H2/t13-,15+,17+,18-,21+/m1/s1 |
|---|
| InChI Key | JPUKWEQWGBDDQB-DTGCRPNFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyranones and derivatives. Pyranones and derivatives are compounds containing a pyran ring which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrans |
|---|
| Sub Class | Pyranones and derivatives |
|---|
| Direct Parent | Pyranones and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl aryl ether
- Pyranone
- Heteroaromatic compound
- Vinylogous ester
- Tetrahydrofuran
- Tertiary alcohol
- 1,2-diol
- Lactone
- Secondary alcohol
- Dialkyl ether
- Ether
- Oxacycle
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000j-0190800000-d3b297eb0fe481caa10a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0190000000-1e520254d0769f8f845e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05n0-3590000000-7f6360b543570fc55716 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-1151900000-879fdf0c200d1a8caf5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1190200000-8b8be60a1a225937d8d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3590000000-22efe60043282fcdf379 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090200000-706a2cc703dc724225bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000k-0090900000-437e416a439402c510ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0090000000-03ec6f5113b2bb58d5cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000900000-84741e5bcc8a2704c4e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0050900000-bcc3a3527efd34a15656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0090000000-d4a9b7661844b5b3c308 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030864 |
|---|
| FooDB ID | FDB004135 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00005137 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-7260 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Trifolin |
|---|
| Chemspider ID | 10306173 |
|---|
| ChEBI ID | 31742 |
|---|
| PubChem Compound ID | 5282149 |
|---|
| Kegg Compound ID | C12626 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|