| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:22:45 UTC |
|---|
| Update Date | 2016-11-09 01:18:05 UTC |
|---|
| Accession Number | CHEM025407 |
|---|
| Identification |
|---|
| Common Name | 3-N-Butyl-4,5-dihydrophthalide |
|---|
| Class | Small Molecule |
|---|
| Description | 3-n-butyl-4,5-dihydrophthalide, also known as nbpa or sedanenolide, is a member of the class of compounds known as isobenzofurans. Isobenzofurans are organic aromatic compounds containing an isobenzofuran moiety. 3-n-butyl-4,5-dihydrophthalide is practically insoluble (in water) and an extremely weak acidic compound (based on its pKa). 3-n-butyl-4,5-dihydrophthalide is a herbal tasting compound found in dill, lovage, parsley, and wild celery, which makes 3-n-butyl-4,5-dihydrophthalide a potential biomarker for the consumption of these food products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
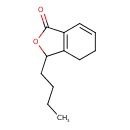
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| NBPA | MeSH | | 3-N-Butyl-4,5-dihydrophthalide, (S)-isomer | MeSH | | Senkyunolide | MeSH | | Sedanenolide | MeSH | | Senkyunolide a | MeSH |
|
|---|
| Chemical Formula | C12H16O2 |
|---|
| Average Molecular Mass | 192.254 g/mol |
|---|
| Monoisotopic Mass | 192.115 g/mol |
|---|
| CAS Registry Number | 62006-39-7 |
|---|
| IUPAC Name | 3-butyl-1,3,4,5-tetrahydro-2-benzofuran-1-one |
|---|
| Traditional Name | 3-butyl-4,5-dihydro-3H-2-benzofuran-1-one |
|---|
| SMILES | CCCCC1OC(=O)C2=C1CCC=C2 |
|---|
| InChI Identifier | InChI=1S/C12H16O2/c1-2-3-8-11-9-6-4-5-7-10(9)12(13)14-11/h5,7,11H,2-4,6,8H2,1H3 |
|---|
| InChI Key | ZPIKVDODKLJKIN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isobenzofurans. These are organic aromatic compounds containing an isobenzofuran moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isobenzofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Isobenzofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isobenzofuran
- 2-furanone
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Dihydrofuran
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-9800000000-23619f15e65aaf35ec7b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1900000000-a1158f039671b5997791 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000f-4900000000-b662d4b24ab550bdc49e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktf-9200000000-4895b56617eb5732e535 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-c22a777084b86bdcc0ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-1900000000-7420f09891f2bf426f6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-9700000000-45de4fd4ff3df8020719 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0258235 |
|---|
| FooDB ID | FDB003895 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 151725 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|