| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:22:33 UTC |
|---|
| Update Date | 2016-11-09 01:18:04 UTC |
|---|
| Accession Number | CHEM025401 |
|---|
| Identification |
|---|
| Common Name | 14-Hydroxy-25-desoxyrollinicin |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
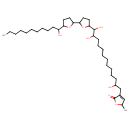
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C37H66O8 |
|---|
| Average Molecular Mass | 638.915 g/mol |
|---|
| Monoisotopic Mass | 638.476 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-methyl-3-(2,12,13-trihydroxy-13-{5-[5-(1-hydroxyundecyl)oxolan-2-yl]oxolan-2-yl}tridecyl)-2,5-dihydrofuran-2-one |
|---|
| Traditional Name | 5-methyl-3-(2,12,13-trihydroxy-13-{5-[5-(1-hydroxyundecyl)oxolan-2-yl]oxolan-2-yl}tridecyl)-5H-furan-2-one |
|---|
| SMILES | CCCCCCCCCCC(O)C1CCC(O1)C1CCC(O1)C(O)C(O)CCCCCCCCCC(O)CC1=CC(C)OC1=O |
|---|
| InChI Identifier | InChI=1S/C37H66O8/c1-3-4-5-6-7-10-13-16-19-30(39)32-21-22-33(44-32)34-23-24-35(45-34)36(41)31(40)20-17-14-11-8-9-12-15-18-29(38)26-28-25-27(2)43-37(28)42/h25,27,29-36,38-41H,3-24,26H2,1-2H3 |
|---|
| InChI Key | HCDACRMLTKJUNU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as annonaceous acetogenins. These are waxy derivatives of fatty acids (usually C32 or C34), containing a terminal carboxylic acid combined with a 2-propanol unit at the C-2 position to form a methyl- substituted alpha,beta-unsaturated-gamma-lactone. One of their interesting structural features is a single, adjacent, or nonadjacent tetrahydrofuran (THF) or tetrahydropyran (THP) system with one or two flanking hydroxyl group(s) at the center of a long hydrocarbon chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
| Direct Parent | Annonaceous acetogenins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Annonaceae acetogenin skeleton
- Long chain fatty alcohol
- 2-furanone
- Dihydrofuran
- Tetrahydrofuran
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Lactone
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Oxacycle
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Polyol
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0021009000-7dca35c57d22ff53b949 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fdo-1931233000-1f94c4c2f44ea008dd6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ry-9842110000-0dc2c2321d91552ef121 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1104029000-775edfad69e0e75dc6dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9233124000-f5d455a22d5c88795646 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-040r-7396231000-3080f442306163dcfc6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0kmr-1101139000-a650493faf46b1af08d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4r-7202049000-a78249ff0b6b7e60b5f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05pp-9300000000-677570e41b60d45e5207 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-3201109000-fa620b3e8ede077a1eb1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-9625356000-194e20639a07723cfc85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9306311000-1ff1fbbe908d6f3587f8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302246 |
|---|
| FooDB ID | FDB003882 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|