| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:22:30 UTC |
|---|
| Update Date | 2016-11-09 01:18:04 UTC |
|---|
| Accession Number | CHEM025399 |
|---|
| Identification |
|---|
| Common Name | Vicenin |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
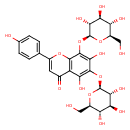
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C27H30O17 |
|---|
| Average Molecular Mass | 626.517 g/mol |
|---|
| Monoisotopic Mass | 626.148 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-bis({[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})-4H-chromen-4-one |
|---|
| Traditional Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-bis({[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})chromen-4-one |
|---|
| SMILES | OC[C@H]1O[C@@H](OC2=C(O)C3=C(OC(=CC3=O)C3=CC=C(O)C=C3)C(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)=C2O)[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C27H30O17/c28-6-12-15(32)18(35)20(37)26(41-12)43-24-17(34)14-10(31)5-11(8-1-3-9(30)4-2-8)40-23(14)25(22(24)39)44-27-21(38)19(36)16(33)13(7-29)42-27/h1-5,12-13,15-16,18-21,26-30,32-39H,6-7H2/t12-,13-,15-,16-,18+,19+,20-,21-,26+,27+/m1/s1 |
|---|
| InChI Key | CSNXTSWTBUEIJB-PXJYGDGDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-8-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C8-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-8-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavonoid-8-o-glycoside
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Flavone
- Hydroxyflavonoid
- Phenolic glycoside
- Hexose monosaccharide
- Chromone
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Pyranone
- Pyran
- Monocyclic benzene moiety
- Oxane
- Monosaccharide
- Benzenoid
- Heteroaromatic compound
- Vinylogous acid
- Secondary alcohol
- Polyol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Primary alcohol
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05mk-0001904000-7b09679a299ba6ff3a0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udj-0109800000-244a68676fdc4370fed1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1339500000-406fdd17a52ee60092d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01t9-1203829000-85156496326abaaba5f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-1332911000-deb48fe6859dc8b51c57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01td-4966300000-11b5f4fc9d28338929e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000009000-ab1396fbffcfda32d16e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000009000-ab1396fbffcfda32d16e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-0401096000-995855b46c5f5aa14673 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000009000-5588ff11777ec050379d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0000009000-08b76504c9641b9fa512 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0900252000-31595fb32b8761dd9e07 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0302244 |
|---|
| FooDB ID | FDB003879 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24610834 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5315206 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|