| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:19:31 UTC |
|---|
| Update Date | 2016-11-09 01:18:03 UTC |
|---|
| Accession Number | CHEM025328 |
|---|
| Identification |
|---|
| Common Name | F-Chlorogenin |
|---|
| Class | Small Molecule |
|---|
| Description | F-chlorogenin is a member of the class of compounds known as triterpenoids. Triterpenoids are terpene molecules containing six isoprene units. F-chlorogenin is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). F-chlorogenin can be found in soft-necked garlic, which makes F-chlorogenin a potential biomarker for the consumption of this food product. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
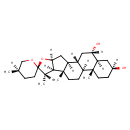
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C27H44O4 |
|---|
| Average Molecular Mass | 432.645 g/mol |
|---|
| Monoisotopic Mass | 432.324 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1'R,2R,2'S,4'S,5R,7'S,8'R,9'S,12'S,13'R,16'S,18'S,19'S)-5,7',9',13'-tetramethyl-5'-oxaspiro[oxane-2,6'-pentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icosane]-16',19'-diol |
|---|
| Traditional Name | (1'R,2R,2'S,4'S,5R,7'S,8'R,9'S,12'S,13'R,16'S,18'S,19'S)-5,7',9',13'-tetramethyl-5'-oxaspiro[oxane-2,6'-pentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icosane]-16',19'-diol |
|---|
| SMILES | [H][C@]12C[C@@]3([H])[C@]4([H])C[C@]([H])(O)[C@@]5([H])C[C@@]([H])(O)CC[C@]5(C)[C@@]4([H])CC[C@]3(C)[C@@]1([H])[C@]([H])(C)[C@@]1(CC[C@@]([H])(C)CO1)O2 |
|---|
| InChI Identifier | InChI=1S/C27H44O4/c1-15-5-10-27(30-14-15)16(2)24-23(31-27)13-20-18-12-22(29)21-11-17(28)6-8-25(21,3)19(18)7-9-26(20,24)4/h15-24,28-29H,5-14H2,1-4H3/t15-,16+,17+,18-,19+,20+,21-,22+,23+,24+,25-,26+,27-/m1/s1 |
|---|
| InChI Key | PZNPHSFXILSZTM-JUGSJECZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Spirostane skeleton
- 3-hydroxysteroid
- 6-hydroxysteroid
- Hydroxysteroid
- 3-beta-hydroxysteroid
- Steroid
- Ketal
- Oxane
- Tetrahydrofuran
- Cyclic alcohol
- Secondary alcohol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Organic oxygen compound
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-3025900000-005759ee34a681e549c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xs-4093300000-c8137ee3fc1b334139ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9056000000-4329390a54f0bb28c320 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-4001900000-89737b67feb56bae786d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02u0-2009500000-c501c6c959f065d4af18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9006000000-e7064d42c03d8f7f091a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0020900000-a800e68f4cb8dd3f2b49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00su-0194500000-d9808e4c7f9f46317672 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-2490000000-51fb95cc3ca90d3ba853 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-f99193fc2ae16b7de32c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000900000-f99193fc2ae16b7de32c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-0113900000-97d177c9a0f2797d7b9c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB003600 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12303065 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|