| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:19:22 UTC |
|---|
| Update Date | 2016-11-09 01:18:03 UTC |
|---|
| Accession Number | CHEM025323 |
|---|
| Identification |
|---|
| Common Name | Dimethyl thiosulfinate |
|---|
| Class | Small Molecule |
|---|
| Description | S-Methyl methanesulfinothioate is found in garden onion. S-Methyl methanesulfinothioate is a constituent of Allium species. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
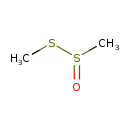
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| S-Methyl methanesulfinothioic acid | Generator | | S-Methyl methanesulphinothioate | Generator | | S-Methyl methanesulphinothioic acid | Generator | | S-Methylmethane thiosulfinate | ChEMBL, HMDB | | S-Methylmethane thiosulfinic acid | Generator, HMDB | | S-Methylmethane thiosulphinate | Generator, HMDB | | S-Methylmethane thiosulphinic acid | Generator, HMDB | | Dimethyl thiosulfinate | HMDB | | Dimethyldisulfide, S-oxide | HMDB | | Methanesulfinic acid, thio-, S-methyl ester (6ci,7ci,8ci) | HMDB | | Methanesulfinothioic acid, S-methyl ester | HMDB | | Methyl methane thiosulphinate | HMDB | | Methyl methanethiosulfinate | HMDB, MeSH | | S-Methyl methanethiosulfinate | HMDB | | S-Methyl thiomethanesulfinate | HMDB | | Methyl methanethiosulfinate, (+-)-isomer | MeSH, HMDB | | Methyl methane thiosulfinate | MeSH, HMDB | | Methyl methanethiosulfinate, (R)-isomer | MeSH, HMDB | | Methyl methanethiosulfinate, (S)-isomer | MeSH, HMDB | | (Methanesulphinylsulphanyl)methane | Generator |
|
|---|
| Chemical Formula | C2H6OS2 |
|---|
| Average Molecular Mass | 110.198 g/mol |
|---|
| Monoisotopic Mass | 109.986 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (methanesulfinylsulfanyl)methane |
|---|
| Traditional Name | (methanesulfinylsulfanyl)methane |
|---|
| SMILES | CSS(C)=O |
|---|
| InChI Identifier | InChI=1S/C2H6OS2/c1-4-5(2)3/h1-2H3 |
|---|
| InChI Key | RRGUMJYEQDVBFP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiosulfinic acid esters. These are organic compounds containing an ester of thiosulfinic acid with the general structure RS(=S)OR' (R, R'=alkyl, aryl). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Thiosulfinic acid esters |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Thiosulfinic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiosulfinic acid ester
- Sulfenyl compound
- Sulfinyl compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9000000000-bc9e61b219b5a9e780ac | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-3900000000-e8d828312433b069cbef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-9400000000-5fa77d797eec5331dbe5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-428c4c26ab83671c9d2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-6900000000-8bc1b221382ad9419698 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-256af55685110e7838d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dl-9000000000-6a96e05da8b14a843afe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9000000000-5cffa5b946acfe767979 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9000000000-1d42c13b28fa1560d546 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-9000000000-feea4d148d614471a900 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9600000000-ffec4a8c2800a42b0816 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9000000000-e58f33ae7652a2ec5388 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-9000000000-a44d2892558d1a7f70fd | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032739 |
|---|
| FooDB ID | FDB010701 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 85904 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 95200 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|