| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:16:49 UTC |
|---|
| Update Date | 2016-11-09 01:18:02 UTC |
|---|
| Accession Number | CHEM025265 |
|---|
| Identification |
|---|
| Common Name | (3S,4S,6R,7S)-1,10-Bisaboladiene-3,4-diol |
|---|
| Class | Small Molecule |
|---|
| Description | (3S,4S,6R,7S)-1,10-Bisaboladiene-3,4-diol is found in herbs and spices. (3S,4S,6R,7S)-1,10-Bisaboladiene-3,4-diol is a constituent of Curcuma longa (turmeric) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
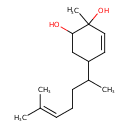
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methyl-2,6-diphenyl-4-piperidinone O-benzyloxime | HMDB | | [1S-[1alpha,2beta,5beta(R*)]]-5-(1,5-dimethyl-4-hexenyl)-2-methyl-3-cyclohexene-1,2-diol | HMDB |
|
|---|
| Chemical Formula | C15H26O2 |
|---|
| Average Molecular Mass | 238.366 g/mol |
|---|
| Monoisotopic Mass | 238.193 g/mol |
|---|
| CAS Registry Number | 129673-87-6 |
|---|
| IUPAC Name | 2-methyl-5-(6-methylhept-5-en-2-yl)cyclohex-3-ene-1,2-diol |
|---|
| Traditional Name | 2-methyl-5-(6-methylhept-5-en-2-yl)cyclohex-3-ene-1,2-diol |
|---|
| SMILES | CC(CCC=C(C)C)C1CC(O)C(C)(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C15H26O2/c1-11(2)6-5-7-12(3)13-8-9-15(4,17)14(16)10-13/h6,8-9,12-14,16-17H,5,7,10H2,1-4H3 |
|---|
| InChI Key | YRFJMOGROZTYPC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bisabolane sesquiterpenoid
- Sesquiterpenoid
- Tertiary alcohol
- Secondary alcohol
- 1,2-diol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dl-6950000000-aec464bb0bfb6fb3adaa | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014i-9356000000-6511e3966926f2550d42 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0390000000-9a79fdb646198dcbde27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00s9-3940000000-107c592b44f6559a9430 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9300000000-8b3bdea85b2a3e51b1b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-2d9862bd182907655fdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0190000000-a165983a3fa62caa4dce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0mba-2950000000-3686922697c6214ee734 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-9410000000-f74f68252ddcc2b11090 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05nf-9200000000-4cd0499b1deba324acd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9100000000-82d49c40c0a5747be5ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-0090000000-a0fb6aa48a0bdbeb0a6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0190000000-4c027da978165f10f253 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-2890000000-8dd3811ab100abb9b669 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031383 |
|---|
| FooDB ID | FDB003450 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00011598 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24022800 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14633005 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|