| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:11:16 UTC |
|---|
| Update Date | 2016-11-09 01:18:01 UTC |
|---|
| Accession Number | CHEM025165 |
|---|
| Identification |
|---|
| Common Name | N-Isobutyl-2,4,8,10,12-tetradecapentaenamide |
|---|
| Class | Small Molecule |
|---|
| Description | N-Isobutyl-2,4,8,10,12-tetradecapentaenamide is found in herbs and spices. N-Isobutyl-2,4,8,10,12-tetradecapentaenamide is a constituent of Zanthoxylum piperitum (Japanese pepper tree) and other Zanthoxylum species |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
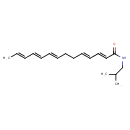
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| g-Sanshool | HMDB |
|
|---|
| Chemical Formula | C18H27NO |
|---|
| Average Molecular Mass | 273.413 g/mol |
|---|
| Monoisotopic Mass | 273.209 g/mol |
|---|
| CAS Registry Number | 78886-65-4 |
|---|
| IUPAC Name | (2E,4E,8E,10E,12E)-N-(2-methylpropyl)tetradeca-2,4,8,10,12-pentaenamide |
|---|
| Traditional Name | (2E,4E,8E,10E,12E)-N-(2-methylpropyl)tetradeca-2,4,8,10,12-pentaenamide |
|---|
| SMILES | C\C=C\C=C\C=C\CC\C=C\C=C\C(=O)NCC(C)C |
|---|
| InChI Identifier | InChI=1S/C18H27NO/c1-4-5-6-7-8-9-10-11-12-13-14-15-18(20)19-16-17(2)3/h4-9,12-15,17H,10-11,16H2,1-3H3,(H,19,20)/b5-4+,7-6+,9-8+,13-12+,15-14+ |
|---|
| InChI Key | KVUKDCFEXVWYBN-FMBIJHKPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl amines. N-acyl amines are compounds containing a fatty acid moiety linked to an amine group through an ester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty amides |
|---|
| Direct Parent | N-acyl amines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-amine
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-6950000000-0727b5a358351fcb8442 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9030000000-aaff8a67cbd39b4115b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000000000-e2934d2bfd0a792a9f60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9100000000-921d722ebf9ced984cd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-ec7cca8f3a30403efd86 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-4690000000-512363cddac8e7a76bae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-9610000000-62acf4fe7c61338aa9fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-4690000000-c3db78701fe88dfa2b8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-9110000000-9ecca852880d90639495 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9300000000-d2ce3cf2418365f6d96e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0390000000-aca37aa4bfb04b4d4236 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-2970000000-315b83660c1cddf75132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-6900000000-79835fe9c67f1694c5e8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031184 |
|---|
| FooDB ID | FDB003203 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00039254 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4477068 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5318518 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|