| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:11:09 UTC |
|---|
| Update Date | 2016-11-09 01:18:01 UTC |
|---|
| Accession Number | CHEM025161 |
|---|
| Identification |
|---|
| Common Name | (E,E)-2,4-Hexadienedial |
|---|
| Class | Small Molecule |
|---|
| Description | (E,E)-2,4-Hexadienedial is found in cereals and cereal products. (E,E)-2,4-Hexadienedial is a stress metabolite isolated from the leaves of the pseudocereal Chenopodium albu |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
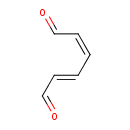
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e,e)-Muconaldehyde | HMDB | | 2,4-Hexadienedial | HMDB | | Hexa-2,4-dienedial | HMDB | | Muconaldehyde | HMDB | | Mucondialdehyde | HMDB | | Muconic aldehyde | HMDB | | Muconic dialdehyde | HMDB | | trans,trans-Muconaldehyde | HMDB |
|
|---|
| Chemical Formula | C6H6O2 |
|---|
| Average Molecular Mass | 110.111 g/mol |
|---|
| Monoisotopic Mass | 110.037 g/mol |
|---|
| CAS Registry Number | 18409-46-6 |
|---|
| IUPAC Name | (2Z,4E)-hexa-2,4-dienedial |
|---|
| Traditional Name | (2Z,4E)-hexa-2,4-dienedial |
|---|
| SMILES | O=C\C=C\C=C/C=O |
|---|
| InChI Identifier | InChI=1S/C6H6O2/c7-5-3-1-2-4-6-8/h1-6H/b3-1-,4-2+ |
|---|
| InChI Key | NDCAAPXLWRAESY-HSFFGMMNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain aldehydes. These are an aldehyde with a chain length containing between 6 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Medium-chain aldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain aldehyde
- Enal
- Alpha,beta-unsaturated aldehyde
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03e9-9500000000-a02f320ad7186601dbe4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-4900000000-bc0dd58197a179a6e1fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9700000000-a6767b8127546cb0d5ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldi-9000000000-11ea80c8000377efd5ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-e9d27d5940f1287154fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3900000000-caf8c89449d5fd8e6275 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-38688207395fd159bacb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-9000000000-798691e72d5526c8334a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016u-9000000000-fd4656cb2adf183660a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-e23f434ac8b62cca7761 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a59-5900000000-38bfdc0998243a815e64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fef-9000000000-2bab49d1e96f51dfb727 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0j4i-9000000000-c5b64b272cd69752a8b3 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031180 |
|---|
| FooDB ID | FDB003199 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9587244 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11412357 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Henderson AP, Bleasdale C, Delaney K, Lindstrom AB, Rappaport SM, Waidyanatha S, Watson WP, Golding BT: Evidence for the formation of Michael adducts from reactions of (E,E)-muconaldehyde with glutathione and other thiols. Bioorg Chem. 2005 Oct;33(5):363-73. Epub 2005 Jul 11. | | 2. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|